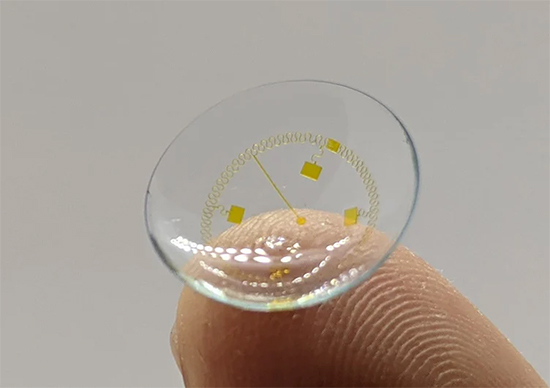
 Image courtesy of InWith Corp |
According to the website, “Our patented platform enables a multiverse of applications within a single contact lens, from XR to tunable focus, to biosensors …” With tunable focus, the company says the lenses can change dioptric power automatically to enhance focus, particularly for myopes and presbyopes, and may have an application for IOLs. Biosensors could be used for health monitoring. The augmented reality feature provides real time information to the wearer, such as directions, weather, local amenities; anything from a smartphone can be displayed in the field of vision. And the extended reality feature brings the viewer into the immersive experience of the metaverse. As for powering the lenses, they have built-in microchips that harness the energy created by blinking.
The website notes CEO and co-inventor Michael Hayes is a “veteran executive and entrepreneur in the field of Technologies and Health Sciences. Mr. Hayes holds inventorship on several granted US technology patents including the Peizoelectric Energy Harvesting Contact Lens, Piezoelectric Sensor for Vision Correction, Medical Devices with Predefined Spaces, Elastic Circuit, System for Contact Lens Wireless Communications, and pending US patents for Adaptive Focus, Method for Imaging Lens and Methods for Artificial Image Spacing.” (Piezoelectricity is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied mechanical stress – Wikipedia.)
InWith expects to obtain FDA Breakthrough Clearance this year. The voluntary program is designed to streamline the market clearance/approval process for certain medical devices and device-led combination products in the United States. InWith plans to have the lenses available soon after approval is granted. The lenses are one-day disposables, eliminating issues with cleaning and storage, and are estimated to cost about twice as much as standard one-day disposables, although the cost is expected to decline with increased demand and manufacturing capabilities.
Contact lenses that only correct vision? We could see so much more this year! Learn more about the future of contact lenses for information and medical monitoring with our CE, Therapeutic Contact Lenses and Beyond, at 2020mag.com/ce.













