
Photographed by Iris Johnson; Frame: Lacoste Tween Collection 3803 from Marchon Eyewear
By Barry Santini
Within the last 20 years, both the prevalence of myopia and its rate of increase within the world’s population are rising. It is estimated that 33 percent of the U.S. population is myopic—defined as greater than -0.50D—and can range up to 80 percent in some Asian countries like Singapore and Taiwan. Particularly in the case of school-aged children ages 7 to 17, many industrialized areas of the world are experiencing rates of increase in myopia approaching 50 percent, a number that cannot be explained by genetic or environmental factors alone.
As a group, young people with myopia are at risk. The negative cognitive, neurological, social, behavioral and economic effects that often accompany uncorrected myopia can have long-term, life-altering impact—especially if their visual impairment remains latent or goes untreated. This is compounded by two facts: Children often have little awareness of their own vision deficiencies, and they also lack the ability to clearly articulate the exact nature of their vision problems.
WHY CONTROL MYOPIA IN CHILDREN?
Controlling myopia in children is important. According to the World Health Organization, whose global health initiative currently places particular emphasis on correcting refractive conditions early, children whose myopia progresses beyond 5 diopters can become adults who are more predisposed to developing glaucoma, cataracts, macular degeneration, retinal holes and tears, as well as detachments of vitreous and retina. And while farsightedness is almost always present at birth, infantile hyperopia decreases in magnitude for most by age 4, while by age 15, almost 15 percent of children have developed some degree of myopia.
According to noted myopia researcher Jeffrey Cooper, OD, the prevalence of myopia in young American adults has increased from 25 percent in 1971 and 1972 to over 41 percent in the period between 1999 and 2004. In Taiwan, the incidence of myopia affects between 60 percent and 80 percent of young adults, and around the world averages 50 percent or more in adolescents—providing strong evidence that something more fundamental than local environmental factors or simple genetics are at work in influencing myopic progression.
BIG CONSEQUENCES
The long-term consequences of neglecting our children’s visual health are manifold and significant. In a 2008 study titled “A Call to Action” underwritten by the Essilor Vision Foundation, Joel N. Zaba, OD, describes the consequences of being complacent about a child’s vision error: “Children with undiagnosed and untreated vision problems grow up to become adults with undiagnosed and untreated vision problems. The failure to detect and treat vision disorders in children affects, among other things, such issues as childhood development, learning performance, self-esteem, social-emotional behavior, academic achievement, high school drop-out rates and juvenile delinquency. From the standpoint of society in general, the failure to detect and treat children’s vision disorders affects the rates of adult criminality, literacy and labor productivity.”
POOR GATEKEEPING
Along with the discovery that myopia is rapidly increasing in our young population, we are beginning to recognize how insubstantial our gatekeeping has been in safeguarding children’s eye health. Although we know that 80 percent of what a child learns comes through their eyes, our society has been slow to institute the changes necessary for improving children’s visual performance. To illustrate this, here are 10 quick facts regarding myopia and the general eye health of children:
1. Only 7 percent of children receive a complete eye exam by the first grade.
2. Up to 66 percent of all children receive no type of preventive vision care, including simple screenings, before they enter the first grade.
3. Only 50 percent have had a complete eye exam by the time they graduate high school.
4. Twenty-five percent of children have a vision problem that interferes with their ability to learn.
5. School screenings are only effective in identifying 5 percent of vision problems.
6. Up to 67 percent of children who fail a school screening do not receive a follow-up exam by an eye doctor.
7. Annually, about 7.5 million children who are visually impaired go untreated.
8. The mean annual myopic progression is between 0.50D - 1.00D.
9. Myopic onset begins around age 6 and dramatically slows around age 16.
10. A 0.25D annual rate of myopic progression is considered clinically relevant.
In the U.S., the National Parent Teacher Organization estimates that more than 10 million children suffer from vision problems that can result in poor academic performance. But now, with the advent of the Affordable Care Act, we have taken the first steps to address the deficits in our vision gatekeeping. Under the ACA, eligible children are now fully covered for yearly eye health exams up to the age of 19. Because ECPs will now begin to see far more children passing through their doors far more frequently, they’ll need to bone up on the latest theories, research and treatments about arresting myopic progression, a new and essential part of their continuing education.
WHAT’S CAUSING THE INCREASE IN MYOPIA?
Here the experts rarely all agree. Most feel that parental genetics are important in determining whether any individual becomes myopic, but in view of the worldwide increase in myopia, researchers often place genetics behind these common suspects:
1. An increase in the amount of time children spend on close-focus activities, such as television, studying, computer and video games.
2. An increase in the amount of time spent using mobile devices, such as smartphones and tablets.
3. A decrease in the amount of outdoor
activities under bright sunlight.
4. The newly-recognized importance that peripheral retinal blur can initiate processes resulting in increased axial growth.
Let’s look at these factors one by one:
A. Increase in close focus activities—Although not universally agreed upon, many myopia experts feel that the increased accommodative tension encountered during sustained close focus tasks weakens aspects of the scleral attachment to the ciliary body, thereby allowing it to stretch or elongate.
B. Increased use of mobile devices—These devices, which are typically held 3 to 4 inches closer to the eye than the normal distance of 16 inches, place additional accommodative and convergent demands on the eye. It is also thought that the light emissive nature of mobile device screens may produce a negative compounding effect on the peripheral retinal images during close tasks and perhaps stimulate axial growth.
C. Decreased outdoor activities—Here theories infer that exposure to sunlight narrows the pupil and therefore reduces peripheral blur through the pinhole effect. Other studies suggest that some wavelengths of sunlight have a beneficial effect by increasing retinal dopamine production, which is thought to help meditate axial growth. There appears to be a correlation between the weekly hours children spend in sunlight activities and the degree of myopic progression.
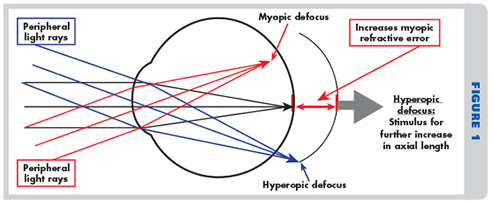
D. Peripheral hyperopic induced blur—The peripheral image shell formed in the eye of a well-corrected or under-corrected myope through the use of best form lenses is hyperopic in nature and therefore found behind the retina. As the peripheral retina’s photosensitive and processing cells have evolved to seek images with the best edge gradients and highest contrast, mechanisms in the eye have been created that automatically try to extend the peripheral retina rearward in order to obtain optimal contrast in the image plane. The end result is an axial elongation of the rear scleral shell, resulting in increase in myopia.
CONTROLLING THE MYOPIC PROGRESSION
When measuring the efficacy of myopia-controlling treatments, today’s researchers use a metric based on quantifying and comparing changes in the axial length of the eye, or more specifically, the change in the depth of the vitreous chamber. Amongst the most important current tenants in research surrounding myopia control is that one cannot completely stop the myopic progression. Below is an overview of the primary treatment avenues that researchers have investigated in their efforts to slow the rate of myopic progression:
Relieving accommodative effort—Historically, both bifocal and progressive spectacle lenses have been employed to reduce the myopic progression by targeting a reduction in accommodative effort, albeit with mixed results. Parents have often intuited that under-correcting the myopic error in spectacles would reduce its progression, but studies have shown that not only is this largely ineffective, it has, in many cases, actually increased the total myopia observed. Alternately, bifocal contact lenses have been shown to offer statically significant reductions in the rate of myopic progression in many studies (see Ortho K below).
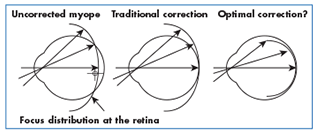
Reducing the neuro-retinal triggers stimulating axial growth—With respect to reducing peripheral hyperopic blur as a key trigger, current research shows that the design of current spectacle lenses, under-correcting a myopic child, having them remove their glasses for close work and even the use on conventional contact lenses all produce a peripheral image shell that is hyperopic in nature. There are special spectacle lenses, however, designed to offer an alternate peripheral aberration correction that have been shown to be a statistically-significant treatment in reducing myopic progression. Carl Zeiss distributes a version of this lens, called MyoVision, in various markets around the world, but not currently the U.S. Additionally, progressive and bifocal contact lenses have been specifically used to target reducing peripheral hyperopic blur and have delivered clinically significant results.
Corneal reshaping—Known variously as Orthokeratology (OK), Ortho-K, Corneal Reshaping Treatment (CRT) and Vision Shaping Treatment (VST), this modality uses a rigid, reverse-geometry design, oxygen-permeable contact lens that is worn overnight. It is designed to flatten the central cornea while steepening the periphery, thereby simultaneously delivering an improved foveal focus while reducing peripheral hyperopic blur. Overall, studies have shown up to a 40 percent reduction in axial length gain through corneal reshaping treatment.
Anti-Muscarinic Therapy—Atropine and pirenzepine are also thought to mitigate the action of the neural-retinal growth trigger in the scleral shell. These drugs, however, even in low dosages, sometimes produce unwanted side effects in selected individuals. Their long-term efficacy for use with children is unknown.
THE EVIDENCE IS CLEAR
In view of the documented negative health, behavioral and economic impact portended by the worldwide increase in the incidence of myopia, it is essential that all eyecare professionals and staff, from eye doctor s to health care technicians to even dispensary personnel, keep up with the latest information on controlling myopic progression in children. Amongst the extensive array of studies, the following stand out and merit your attention: the Correction of Myopia Evaluation Trail (COMET); Children’s Overnight Orthokeratology Investigation (COOKI); The Contact Lens and Myopia Progression study (CLAMP); the Corneal Reshaping and Yearly Observation of Nearsightedness study (CRAYON); the Longitudinal Orthokeratology Research in Children study (LORIC) and the forthcoming Stabilization of Myopia by Accelerated Reshaping Technique trial (SMART).
Although most of the treatment modalities mentioned above have not yet received FDA approval, they have been proven to be at least statistically, if not clinically effective. As motivated parents have easy access to information on the Web regarding myopia control, ECPs are strongly encouraged to begin supplementing their continuing education with the website
myopiaprevention.org.
Early intervention is recognized as key in producing viable results for reducing myopic progression. During the next few years, as eyecare professionals begin to see more young children in their practice for general screenings and exams, it is important to become proactive about educating parents to be vigilant in safeguarding their child’s vision, particularly if there is no overt symptom or complaint. More than ever, today is the best time for every ECP to begin preparing for both the inevitable questions as well as the monetary rewards that will accompany the public’s interest in controlling myopia.
L&T contributing editor Barry Santini is a New York State-licensed optician based in Seaford, N.Y.
Thanks to Dr. Saulius Varnas, Dr. Stephen Silberberg and Dr. Howard Purcell for their help.
The Eye and Myopia
When parents arrive with their teenager in tow for a scheduled eye checkup, an oft repeated question
is revealed: “We came in for our yearly checkup, and we just knew we’d be getting a new Rx again. Doctor, can you tell me why their eyes are getting worse every year?” In order to provide the best answer, a working knowledge of the eye and its development during the first 10 years of life is essential.
All babies are optically farsighted at birth. Although the newborn’s eye has an axial length and globe size averaging 8 mm and 75 percent less than an adult eye, it is compensated by:
1. Their cornea and lens being more steeply curved.
2. Their lens having a greater refractive index, and
3. Their accommodative range being greater.
But what happens to them as their bodies experience the rapid physiological growth normal in the first 10 years of life? How does nature react to coordinate the various optical elements within the eye to help keep an image in focus?
EMMETROPIZATION
The process of coordinating the refractive power of the cornea, the lens and the axial length of the eye in order to present a sharp image of a distant object on the retina is called emmetropization.
As the eye grows during the first 10 years of life, the natural widening of the globe’s structural collagen more or less pulls on both the cornea and through its ciliary attachment to the lens capsule, the zonules and the lens—thereby flattening their curvatures and reducing refractive power. For example, while the eye’s axial length grows 5 to 6 mm by age 6, the cornea and lens together are shedding a total of 24 diopters of refracting power. Note: The relationship of axial length to equivalent dioptric power is seen in this example. The correlation is approximately 3 diopters for every millimeter of axial length increase. This delicate ballet between corneal curvature, lens power and axial length continues naturally to up and even beyond age 10, where the normal eye reaches approximately 90 percent of its adult size.
THREE NATURAL PROCESSES KEEP FOCUS IN CHECK
When normal eye globe growth is mostly complete by the early teens, the anterior corneal has also generally reached its adult radius of curvature. At this point, nature has but two tools left with which to keep an image sharply focused on the retina: accommodation and axial length adjustment, with the latter innervated by a complex reaction of enzymes on the elastic collagen fibers that make up the scleral shell. Obviously, lens accommodation is effective at moving an image that lies behind the retina to the fovea, an area where nature has conveniently placed the greatest concentration of its most advanced retinal receptors, the color-discerning cones. But accommodation is not effective in relocating an image found in front of the retina. As the eye lacks any real ability to reduce its globe size through natural means, an image found in front of the retina cannot prompt a reduction in axial length. And even if an image is found in perfect focus, recent discoveries have uncovered how the eye’s natural emmetropization process is additionally influenced by photoreceptors outside the foveal surround. The peripheral retina, despite its poor acuity, will send out signals that trigger an increase in the eye’s axial length in situations where the peripheral retinal image lacks stimulation, has low contrast or is hyperopic in position.
THE POWER OF THE PERIPHERAL RETINA
All eyecare professionals have been taught to appreciate how important the fovea and macula are to providing us with the ability to see as sharply as possible. But just outside this zone of densely-packed, high-resolution photoreceptors, there lies an area which is many, many times larger than the macula. And even though this is a region that features less-sophisticated and less densely populated photoreceptors, the peripheral retina contains some important contrast-sensing circuitry essential to our eye’s natural emmetropization process. To best understand the power of the peripheral retina, we’ll review the basics about the mapping of the retina’s different photoreceptor areas, starting within the central fovea and moving outward in the interior of the globe.
Foveola—A 0.35-mm diameter area centered within the fovea centralis of the retina, featuring the greatest density of cones and containing no rods. Its area subtends a 1-degree field of view (FOV). The foveola is capable of seeing the highest degree of visual acuity (20/08), basic on its retinal mosaic density.
Fovea Centralis—A 1 to 1.5-mm diameter area centered within the macula, representing only 1 percent of the total area of the retina. Its area subtends a 3 to 5-degree FOV. The fovea is literally a numerical Game of Fives:
1. 50 percent of all fibers leaving through
the optic nerve are connected to the
fovea centralis.
2. The fovea contains 50 percent of all the
eye’s photoreceptors.
3. The fovea is directly mapped to 50 percent
of the visual cortex.
4. The fovea is found displaced 5 degrees
off the optical axis of the eye.
Macula—A 6-mm diameter area roughly centered in the retina and temporally placed to the optic nerve. It represents just 5 percent of the total area of the retina and subtends a 15 to 18-degree FOV. It features a mix of rods and cones.
 Peripheral Retina—Defined as all the retinal real estate outside the macula, it is separated into the mid-peripheral and far peripheral areas by its globe’s equator. The equator is defined as a circle subtending the largest dimension of the globe, and whose origin is located on the anterior-posterior pole. It represents approximately 67 percent of the globe’s inner surface, making it by far the largest area of the retina. Starting approximately 18 degrees from the fovea, the peripheral retina extends to the ora serrata, the junction between the retina and the ciliary body. The visual acuity of the peripheral retina decreases rapidly as we move away from the macula and is overall, many, many times worse than the fovea. The limits of peripheral retina define an FOV of 100 degrees vertical and 120 degrees horizontal. Comprised of mostly rod photoreceptors, there are additionally present 1 to 3 percent of ganglion cells that also have photoreceptive properties. The ganglion cells found in the mid-peripheral retina layers are thought to be responsible for autonomic management of circadian rhythms and pupillary reflexes.
Peripheral Retina—Defined as all the retinal real estate outside the macula, it is separated into the mid-peripheral and far peripheral areas by its globe’s equator. The equator is defined as a circle subtending the largest dimension of the globe, and whose origin is located on the anterior-posterior pole. It represents approximately 67 percent of the globe’s inner surface, making it by far the largest area of the retina. Starting approximately 18 degrees from the fovea, the peripheral retina extends to the ora serrata, the junction between the retina and the ciliary body. The visual acuity of the peripheral retina decreases rapidly as we move away from the macula and is overall, many, many times worse than the fovea. The limits of peripheral retina define an FOV of 100 degrees vertical and 120 degrees horizontal. Comprised of mostly rod photoreceptors, there are additionally present 1 to 3 percent of ganglion cells that also have photoreceptive properties. The ganglion cells found in the mid-peripheral retina layers are thought to be responsible for autonomic management of circadian rhythms and pupillary reflexes.
 POLAR MAP PROJECTION OF THE EYE
POLAR MAP PROJECTION OF THE EYE
Although most of our visual information comes to us from the macula, it is important to not discount the important contribution to overall light-sensing and processing that the peripheral retina represents. Here retinal cells besides the classic photoreceptors have evolved to contribute in our visual system’s directive to seek and sense contrast, shadow borders and motion. As hunter gatherers, sensing movement in our peripheral vision was essential to finding food and avoiding predators. Although visual acuity here may be low, the contrast and motion-seeking sensitivity of the peripheral retina is important to sensing when an extended image—one that lies outside the macula—is not rendered in optimal contrast and therefore optimal focus.
—BS












