Leiv M. Takle Jr., MD, and C. Robert Bernardino, MD FACS, Atlanta
Community-acquired methicillin Staphylococcus aureus (CA-MRSA) is a rapidly emerging public health problem. There was a time when the medical community was concerned solely with resistant hospital-acquired infections, but community-acquired resistant infections are emerging as a villain on the infectious-disease front. Not only is it important on a public-health scale but also rapidly emerging as a problem in the ophthalmic community as both a diagnostic and therapeutic challenge.
Methicillin-resistant Staphylococcus aureus (MRSA) has traditionally been thought of as a hospital-acquired pathogen, and empiric therapy of community-acquired gram-positive infections could reliably be initiated with a cephalosporin or fluoroquinolone.1 However, several strains of community-acquired MRSA (CA-MRSA) have recently been isolated and have shown resistance to the beta-lactam group of antibiotic therapy.2,3 Unlike its hospital-acquired cousin, CA-MRSA occurs in patients with no exposure to hospitals, and it is just as virulent as the nonresistant strains.3,4 For the ophthalmologist, it is important to maintain a keen sense of awareness of this condition when presented with ocular adnexa/soft tissue infections not responding to first-line therapy. This article will review the history of MRSA, epidemiology, clinical manifestations and treatment options and guidelines for soft tissue infections concerning the ocular adnexa.
History
In the early 1990s, CA-MRSA infections were reported among Australian aboriginals and Native Americans in Canada who had little or no previous contact with health-care facilities.5 Subsequently, clusters or outbreaks of CA-MRSA infections have affected children in day-care centers, native American communities in several states, athletic teams, military personnel, men who have sex with men in Los Angeles and San Francisco, and prison inmates in several states. 6-11
In studies performed between 1997 and 2002, MRSA accounted for a significant proportion of community-acquired S. aureus isolates.10,11 The clinical characteristics of CA-MRSA and hospital-acquired MRSA (HA-MRSA) were compared in a prospective study of 1,100 MRSA infections in
Community strains of MRSA may arise in either of two ways: Hospital strains may be carried into the community, where they then spread person to person; or community MRSA may arise de novo when the methicillin-resistance gene complex is acquired by a methicillin-susceptible strain.13 Anecdotal reports, case series and studies of outbreaks suggest the latter is occurring, but these may not accurately represent strains circulating in the community.
These reports of infection and colonization by strains of MRSA provide compelling evidence that MRSA strains, like penicillinase-producing strains almost 30 years ago, have gained a foothold in the community and are emerging as important outpatient pathogens.14
Definition of CA-MRSA
CA-MRSA infections are distinguished from hospital-acquired MRSA infections when the patient with MRSA meets the criteria in Table 1.15

Epidemiology, Ophthalmic Literature
In 2000, the CDC began working closely with several states with a combined population of about 12 million persons to study the epidemiology of CA-MRSA infections. Information from these studies is helping the CDC understand the nature of the disease, the reasons why people get infected, and the types of research needed to help prevent these infections. These data are being collected in
CA-MRSA appears to be an infection of the young. In a study looking at Minnesota health-care facilities looking specifically at CA-MRSA isolates, the median age of patients was 16 years (range 1 to 78 years), and the most common infection type was skin and soft tissue (84 percent).16 To date, the true incidence of CA-MRSA is unknown, since most studies have characterized this organism in a relatively small group of patients over a short, fixed time interval, however, there is evidence that this organism is on the rise.
The ophthalmic manifestations of this growing pathogen were looked at closely in a prospective case series, a retrospective lab review of 549 MRSA isolates.17 Nine of these isolates were CA-MRSA affecting the eye and orbit. The infections ranged from orbital cellulitis, endogenous endophthalmitis, panophthalmitis and lid abscesses to septic venous thrombosis. Patients were treated with trimethoprim-sulfamethoxazole, rifampin, clindamycin or vancomycin based on microbial sensitivity studies and severity of infection. Seven of these patients had soft tissue infections and required hospitalization, including four lid abscesses ranging from localized infection and preseptal cellulitis to one case of orbital cellulitis resulting in good visual outcome. The treatment of this cohort required debridement of necrotic tissue in addition to a non-b-lactam class of antibiotics.17
Clinical Characteristics
Patients infected with CA-MRSA frequently present with skin or soft tissue infections. For the ophthalmologist this usually presents as a boil, furuncle or abscess and can lead to preseptal and postseptal cellulitis. Cutaneous lesions can be as large as 5 centimeters in diameter and usually spread rapidly to involve the orbit within days. These patients usually are not immunocompromised. Pain and erythema seem to present out of proportion to the severity of the cutaneous lesion and necrosis seems to be a strong indicator of infection with community acquired MRSA. Anecdotally, some patients have thought the infection started with an insect bite or spider bite.
The common secreted virulence factors of Staphylococcus aureus include toxic shock syndrome toxin 1 and several staphylococcal enterotoxins. These toxins cause toxic shock and related illnesses through induction of massive cytokine release, both from macrophages and T cells. Another significant virulence factor in staphylococcal infections is the Panton-Valentine leukocidin (PVL).18 This increasing virulence factor is most often associated with the skin and soft tissue infections and has been shown to confer increased virulence and makes CA-MRSA more easily transmissible and rapidly invasive.19
Treatment
Therapeutic management of this infection has not been well studied and is not well established. For the ophthalmologist, this poses a dilemma on when to start thinking about CA-MRSA in the initial management. Certainly, the spectrum of how CA-MRSA can present can range from uncomplicated skin and soft tissue infections to orbital cellulitis. The therapeutic options should be directed at the specific entity and the clinical suspicion of CA-MRSA should always remain a possibility in the initial differential or the lack of response to first-line treatments.
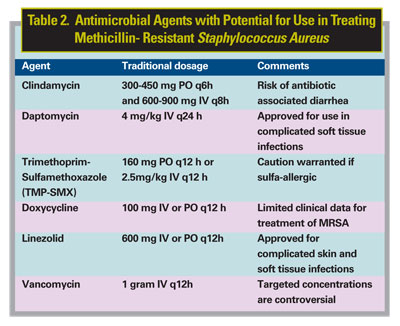
Because CA-MRSA strains tend to be susceptible to a wide variety of non-b-lactam antibiotics, there are options available in the treatment of this organism. However, most potential treatments have not been tested clinically, and their efficacy is therefore unknown. Researchers have expressed renewed interest in the use of clindamycin, tetracyclines, and trimethoprim-sulfamethoxazole (TMP-SMX) in treating MRSA infections, as these drugs generally have activity against CA-MRSA (See Table 2). For severe infections requiring hospitalization together with intravenous antibiotics, vancomycin and newer agents such as linezolid and daptomycin can be feasible options.
Clindamycin remains a possible treatment option for CA-MRSA, as it demonstrates in vitro susceptibility to most MRSA isolates.20 However, inducible resistance and treatment failure have been reported.21 The disk diffusion (D-test) method can detect S. aureus isolates with inducible macrolide-lincosamide-streptogramin B (iMLSB) resistance.22 However, this is a cumbersome procedure, and hospital laboratories have had difficulty applying this method to each S. aureus isolate. Inducible isolates (those with positive results on the D-test) are a source of concern because they may have a heightened rate of mutations, which would enable them to develop resistance to clindamycin during therapy. It is not currently recommended to use clindamycin to treat MRSA infections unless the appropriate D-test for resistance is conducted on the specified isolate according to the guidelines of the National Committee for Clinical Laboratory Standards.22
Trimethoprim-sulfamethoxazole (TMP-SMX) is another antibiotic for treatment of CA-MRSA. Currently, limited clinical studies and patient cases have found TMP-SMX to be useful in treating MRSA infections, but it has shown case-by-case efficacy.23 The reported rate of TMP-SMX resistance in S. aureus is highly variable, but most CA-MRSA strains appear to be susceptible. The sulfa moiety of TMP-SMX, sulfamethoxazole, is bacteriostatic, whereas the trimethoprim component blocks dihydrofolate reductase, thus inhibiting production of metabolically active folic acid. When both agents are used together, TMP-SMX appears to produce a bactericidal effect against most isolates.23
One study investigated the use of TMP-SMX in MRSA bacteremia in intravenous drug users. The investigators reported that TMP-SMX may be considered as an alternative to vancomycin in selected cases of MRSA infection; however, treatment failures were reported.23 Allergic reactions to sulfonamide antibiotics have been reported as high as 10 percent in some patients; therefore, caution is warranted. The development of resistance and the therapeutic use of TMP-SMX in MRSA have not been fully established. However, a small retrospective study suggested that prompt incision and drainage, followed by a two-to-three-week course of TMP-SMX in combination with rifampin, was an effective treatment option for cutaneous infections caused by CA-MRSA.23 Currently it is not recommended to treat with rifampin in a monotherapy modality because of rapid resistance patterns that have emerged.
Minocycline and doxycycline are tetracycline antibiotics that have been used in the past for the treatment of MRSA. Although these agents have been used clinically, limited published data are available on the use of tetracyclines in the treatment of MRSA; therefore, the efficacy of doxycycline and minocycline in the treatment of MRSA have not been established.
Most infectious disease journals report caution when using fluoroquinolones in the treatment of MRSA infections. In the past, there has been gross overuse of these drugs, which has led to acquisition of fluoroquinolone-resistant MRSA. The more recent fluoroquinolones may have a role in the treatment of soft tissue infections, especially CA-MRSA, however there are inadequate studies to support this. More clinical studies are needed before a formal recommendation can be made.
Until the arrival of newer agents, most clinicians would agree that vancomycin, a glycopeptide antibiotic, is the drug of choice for treating serious MRSA infections, such as orbital cellulitis. However, recent reports have described vancomycin-intermediate susceptible S. aureus (VISA) as a newcomer on the scene, and to date vancomycin-resistant S. aureus (VRSA) has been identified in three states.24 These organisms are often not responsive to vancomycin and will soon be the next challenge in this infectious dilemma.
Linezolid, which in 2000 became the first oxazolidinone to be approved by the Food and Drug Administration, has good penetration into skin and soft tissue infections and is available in an oral formulation. Although it is considered a bacteriostatic agent, linezolid has demonstrated effectiveness in skin and soft tissue infections, bacteremia and pneumonia caused by gram-positive bacteria. Linezolid shows activity against MRSA, although S. aureus resistance has been reported.25,26
Daptomycin is a lipopeptide antibiotic. It is approved by the FDA for the treatment of complicated skin and soft tissue infections, including those caused by MRSA. This drug has received much interest because of its activity against multidrug-resistant gram-positive bacteria, especially MRSA. Currently, most of the data exists from animal models but does demonstrate rapid bactericidal activity. Although considered a viable treatment option, its role in the treatment of CA-MRSA has not yet been fully established.
Overall, there appear to be several antimicrobial therapeutic options for treating CA-MRSA infections. However, as stated above, no definitive studies have tested these options clinically.
How Should OphthalmologistsTreat?
For primary soft tissue infections (preseptal) the mainstay of therapy is still b lactam and fluoroquinolone antibiotics. If there is a lack of response to initial treatment, early incision and drainage with gram staining and culture should be performed to categorize the infection agent and determine antibiotic sensitivity. If a resistant organism is encountered with poor response to oral antibiotics, hospitalization and IV antibiotic therapy as well as surgical debridement is warranted. Consultation with an infectious-disease specialist is often helpful, since they may know local antimicrobial resistance patterns and can help direct therapy.
Currently it is not recommended to eradicate the colonization of household contacts but this should be considered if there are recurrent infections or on-going transmission in close contacts. Good hand hygiene through hand washing remains the mainstay of prevention. Lastly, the CDC has outlined specific guidelines for the treatment of soft tissue infections associated with CA-MRSA and above is an abbreviated version of the recommendations, applicable to the ophthalmologist (See Table 3).27
Drs. Takle and Bernardino are at the Emory University School of Medicine, Department of Ophthalmology, Oculoplastics and Orbital Surgery. Contact Dr. Bernardino at 1365B
The authors have no financial interest in any of the subjects discussed in this manuscript.
1. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999-1005.
2. Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis 2001;7:178-82.
3. Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998;339:520-32.
4. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201-5.
5. Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health-care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976-84.
6. Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci USA 2002;99:7687-92.
7. Frank
8. Abudu L, Blair I, Fraise A, Cheng KK. Methicillin-resistant Staphylococcus aureus (MRSA): A community-based prevalence survey. Epidemiol Infect 2001;126:351-6.
9. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus infections among competitive sports participants—
10. Baum SE, Morris JT, Dooley DP, Watson R. Methicillin-resistant Staphylococcus aureus in an adult military beneficiary population lacking risk factors: Susceptibility to orally available agents. Mil Med 2003;168:126-30.
11. Centers for Disease Control and Prevention. Public health dispatch: Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. Can Commun Dis Rep 2003;29:110-12.
12. Naimi TS, LeDell KH, Boxrud DJ, et al. Epidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996-1998. Clin Infect Dis 2001;33:990-6.
13. Mongkolrattanothai K, Boyle S, Kahana MD, Daum RS. Severe Staphylococcus aureus infections caused by clonally related community-acquired methicillin-susceptible and methicillin-resistant isolates. Clin Infect Dis 2003;37:1050-8.
14.
15. Centers for Disease Control and Prevention. Community-associated MRSA. Information from the
16. Naimi TS, LeDell KH, Boxrud DJ, et al. Epidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996-1998. Clin Infect Dis 2001;33:990-6.
17. Rutar, T, Chambers HF, Crawford et.al. Ophthalmic manefestations of infections caused by the USA300 clone of community-associated methicillin-resistant staphylococcus aureus. Ophthalmology 2006;113:1455-62.
18. Dufour P, Gillet Y, Bes M, et al. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: Emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis 2002;35:819-24.
19. Baggett HC, Hennessy TW, Rudolph K, et al. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska. J Infect Dis 2004;189:1565
20. Frank AL, Marcinak JF, Mangat PD, et al. Clindamycin treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr Infect Dis J 2002;21:530-4.
21. Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis 2003;37:1257-60.
22. Fiebelkorn KR, Crawford SA, McElmeel ML, Jorgensen JH. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J Clin Microbiol 2003;41:4740-4.
23. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med 1992;117:390-8.
24. Rybak MJ, Akins RL. Emergence of methicillin-resistant Staphylococcus aureus with intermediate glycopeptide resistance: Clinical significance and treatment options. Drugs 2001;61:1-7.
25. Pillai SK, Sakoulas G, Wennersten C, et al. Linezolid resistance in Staphylococcus aureus: Characterization and stability of resistant phenotype. J Infect Dis 2002;186:1603-7.
26. Leclercq R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin Infect Dis 2002;34:482-92.
27. Centers for Disease Control and Prevention. Community-associated MRSA. Information from the











