According to researchers in
This retrospective chart review included cases from 10 corneal and refractive disease practices. All charts were reviewed, surgeons questioned and patients interviewed for risk factors related to MRSA. All patients underwent microbial culturing and sensitivity testing and were treated with a combination of commercial and fortified antibiotics. All cases occurred between May 2002 and February 2005. A comprehensive literature search was performed to identify all cases of infectious keratitis including MRSA keratitis after refractive surgery.
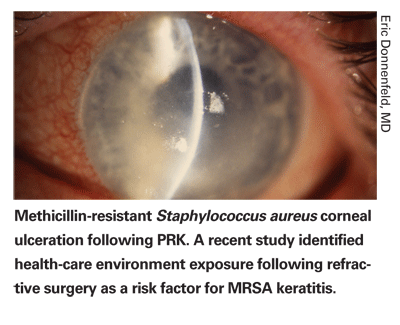
Twelve eyes of 11 patients with a mean age of 43.9 years (range, 24 to 60 years) developed MRSA keratitis after refractive surgery. Eight patients presented following an initial LASIK procedure, and one presented after a LASIK enhancement. Two patients presented after a PRK procedure, and one patient presented with a bilateral infection. The refractive procedure was always first performed in the right eye. Seven infections occurred in the first eye and five were in the second eye. There were seven male and five female patients. Five patients were health-care workers, and four had exposure to a health-care setting. Three denied any exposure to a health-care setting.
All patients presented with a decrease in visual acuity and complaints of pain or irritation in the affected eye. Common signs on slit-lamp biomicroscopy were corneal epithelial defects, focal infiltrates with surrounding edema, conjunctival infection, purulent discharge and hypopyon. All patients were diagnosed with infectious keratitis on presentation and treated with two antibiotics.
According to a computerized literature search, this was the first case series of MRSA infectious keratitis following refractive surgery, the first reports of MRSA after surgery in patients with no known health-care facility exposure, the first report of MRSA after a LASIK enhancement and the first reports of MRSA keratitis after prophylaxis with fourth-generation fluoroquinolones. The researchers say the study is limited by its retrospective nature. The study did not attempt to identify the incidence of MRSA, making it impossible to ascertain the incidence of MRSA in the general population. They suggest studies for the ophthalmic use of linezolid for prophylaxis and treatment of MRSA keratitis.
(Am J Opthalmol 2007;143:629-634)
Solomon R, Donnenfeld ED, Perry HD, Rubinfeld RS, Ehrenhaus M, Wittpenn JR, Solomon KD, Manche EE, Moshirfar M, Matzkin DC, Mozayeni RM, Maloney RK.
Line of Vision Cost in AMD
New pharmacologic therapies for age-related macular degeneration are extremely expensive and some yield marginal visual dividends, according to a researcher in
Key clinical studies establishing the efficacy of various therapeutic AMD treatments were identified and carefully reviewed to quantitate the visual benefit. For comparison, representative treatment studies for common retinal conditions including retinal detachment, macular hole, epiretinal membrane and diabetic retinopathy were also reviewed.
Several parameters to estimate Snellen lines of vision saved were defined and tabulated for each condition. Costs for a regimen of office visits, ancillary testing and treatments were tabulated using Medicare-allowable costs, and costs of visual benefit (per line of vision) for each condition were calculated.
The cost per line of vision saved for AMD therapies ranged from $997 for laser for extrafoveal choroidal neovascularization, to $5,509 for photodynamic therapy for occult lesions, to $12,482 for pegaptanib injections. This compares to $651 for retinal detachment repair, $1,658 for MH repair, $2,411 for ERM peeling, $5,458 for diabetic macular edema laser, $594 for panretinal photocoagulation and $2,984 to $4,178 for diabetic vitrectomy. The costs per line-years ranged from $77 to $1,248 for AMD, and $21 to $194 for the comparison conditions. The proportion of costs for pegaptanib treatment was 17 percent for professional fees and 70 percent for pharmaceutical fees.
Assumptions incorporated in estimating costs for pegaptanib could easily have doubled because second-year costs might approximate first-year costs and the maintenance of treatment effect has not been well-established.
The researcher admits to assumptions that likely underestimate the disparity in costs. Long-term follow-up information for AMD treatment is lacking; the amount of vision preserved by treatments probably does not continue to improve and how well it is maintained awaits confirmation. The nature of diseases can differ; AMD requires more chronic, ongoing care than most of the comparison conditions cited in the study. The point at which treatment may be discontinued is nebulous. He recommends that costs should be considered as they relate to health-care costs for the individual patient and payers, and must be considered in a larger perspective of health-care benefit apportionment.
(Ophthalmology 2007;114:847-854)
Smiddy, WM.
Stromal Bed Quality in LASIK
In LASIK surgery, the IntraLase 30-kHz femtosecond laser produces smoother stromal beds than the IntraLase 15-kHz and the Hansatome 160-µm head using a new blade, according to researchers in
The IntraLase 15- and 30-kHz femtosecond laser and the Hansatome were used to create LASIK flaps in nine unfrozen human globes in three groups of three globes each. All procedures were performed by one surgeon skilled in LASIK.
Group 1 was the control, in which the flaps were created using a Hansatome microkeratome with a 160-µm head. Group 2 consisted of flaps created at the 110-µm thickness setting using the IntraLase 15-kHz and Group 3, of 110-µm flaps using the IntraLase 30-kHz laser.
The flaps and stromal beds were examined by scanning electron microscopy. Surface quality of the SEM images was graded by three masked observers and by using software designed for roughness analysis.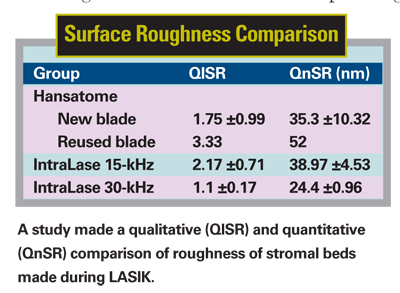
In Group 1, samples cut with the Hansatome 160-µm head and a new blade created a stromal bed without irregularities or chatterlines (qualitative surface roughness [QlSR] = 1.75 ±0.99; quantitative surface roughness [QnSR] = 35.3 ±10.32 nm). The bed was entirely smooth and compact. The sample cut with the reused blade, however, showed a rougher bed than the two previous samples (QlSR = 3.33; QnSR = 52 nm). Group 2 15-kHz IntraLase samples showed a stromal bed without irregularities, without tissue tags and with a smoothness comparable to that of Group 1 (QlSR = 2.17 ±0.71; QnSR = 38.97 ±4.35 nm). There were no statistically significant differences between Group 1 (new blade only) and Group 2 QlSR (p=0.244) and QnSR (p=0.564). Group 3 30-kHz IntraLase samples showed a smoother stromal bed than Group 1 and 2 samples. This was a statistically significant difference with QlSR = 1.1 ±0.17 (p<0.001) and showed borderline significance with QnSR = 24.4 ±0.96 nm (p=0.05).
The researchers state that improvement in blade quality control and tightening of the tolerances of the microkeratome head gap will improve the predictability of flap dimensions, but the basics of flap creation are unchanged with the newer mechanical microkeratomes. They suspect that the morphological features will remain unchanged from those documented in their study.
(Cornea 2007;26:446-451)
Sarayba MA, Ignacio TS, Binder PS, Tran DB.











