Just as bacteria have two sides to them, with the ability to be both pathogenic and helpful to the body,1 antibiotics can also have dual effects: fighting both bacteria and inflammation. While it's intuitive that eradicating the source of infection will reduce inflammation—that's part of healing—antibiotics may also influence the inflammatory immune response independent of whether or not bacteria are present. Though the exact mechanisms by which antibiotics block inflammation are not clearly understood and are still in the process of evaluation, it's clear they have some ability to modulate the immune response. In this article, we'll explore what we currently know about the anti-inflammatory side of antibiotics.
In-vitro and Preclinical Analysis
Unfortunately, clinical research on the anti-inflammatory effects of antibiotics is complicated by the fact that many inflammatory conditions have infectious origins. Research has therefore been relegated to two areas: in-vitro analysis of the inflammatory responses of cells stimulated with pro-inflammatory cytokines, with no infectious involvement; and in-vivo analysis of the ability of various antibiotics to mitigate inflammation. In the latter, inflammation typically has an infectious origin, although the organisms involved aren't always known.
Fluoroquinolones, a class of antibiotics discovered in the 1980s that inhibit DNA gyrase activity, have been shown to have inflammation-mitigating effects. In mice challenged with lipopolysaccharides from the cell membrane of gram-negative bacteria, inflammatory responses were diminished upon treatment with trovafloxacin, ciprofloxacin and tosufloxacin. This study also found that cytokine levels were significantly decreased.2 At the recent meeting of the Association for Research in Vision and Ophthalmology, a poster described the anti-inflammatory effects of besifloxacin, currently in development at Bausch & Lomb (Cavet ME, et al. IOVS 2008;49: ARVO E-Abstract 2500), on corneal epithelial cells in vitro. By stimulating the epithelial cells with the pro-inflammatory cytokine interleukin-1 beta, researchers found that besifloxacin significantly inhibited inflammatory cytokine production in a dose-dependent manner.
Discovered in the 1940s, tetracyclines (which include minocycline and doxycycline) act on bacterial ribosomes, preventing them from effectively translating mRNA into vital proteins. Widely used for anti-infective applications, the tetracyclines are still a class of focus some 60 years after their discovery, largely due to their unique anti-inflammatory properties. Interestingly, their anti-inflammatory mechanism may be related to their anti-infective mechanism, by means of disrupting the translational process from mRNA to protein assembly. The secretion of pro-inflammatory cytokines, including tumor necrosis factor alpha and IL-1b, decreased with respect to tetracycline concentration, while levels of mRNAs encoding for these cytokines weren't affected.3
Multiple studies conducted to identify the tetracyclines' anti-inflammatory mechanisms of action have succeeded in identifying modulation in pro-inflammatory cytokine production, but have not definitively identified a single causal activity. Doxycycline downregulates a wide variety of pro-inflammatory cytokines (interleukins and tumor necrosis factors) and chemokines, which are produced through various mitogen-activated protein kinase pathways, a series of cellular activities that lead to inflammation. These inflammatory cytokines recruit leukocytes to the area of inflammation, leading to exacerbated tissue damage. Doxycycline also downregulates the expression of several pro-inflammatory enzymes, including matrix metalloproteinase and collagenase, which are responsible for the cleaving of extracellular proteins in an inflammatory state.4-6 Similarly, minocycline downregulates a wide variety of pro-inflammatory proteins, including several cytokines, immunoglobins, collagenase and intercellular adhesion molecule-1, a critical surface protein that promotes inflammation and is expressed on leukocytes.5,7,8,9 Studies have shown minocycline decreases nitric oxide, possibly by downregulating nitric oxide synthase, the enzyme that catalyzes the production of nitric oxide, a by-product of the inflammatory cascade in mice macrophages (ex vivo) and rats.10,11
A myriad of studies have shed some light on the underlying mechanism of action of macrolides' anti-inflammatory capabilities. Erythromycin, discovered in the 1950s, inhibits inflammation via downregulation of pro-inflammatory cytokines, chemokines, oxidant production and increased apoptosis of neutrophils.12,13 Clarithromycin, a derivative of erythromycin discovered in the 1970s, prohibits expression of cytokines and ICAM-1.14 Azithromycin, an erythromycin derivative discovered in 1980, not only inhibits mediators secreted by leukocytes, but also increases the percentage of macrophages that phagocytose apoptotic bronchial epithelial cells, possibly due to altered phenotype.15-17 Furthermore, macrolides appear to inhibit NO production by blocking NOS expression in rats.18 As a high level of NO has been associated with inflammation, NO inhibition may be one of multiple anti-inflammatory mechanisms of macrolides.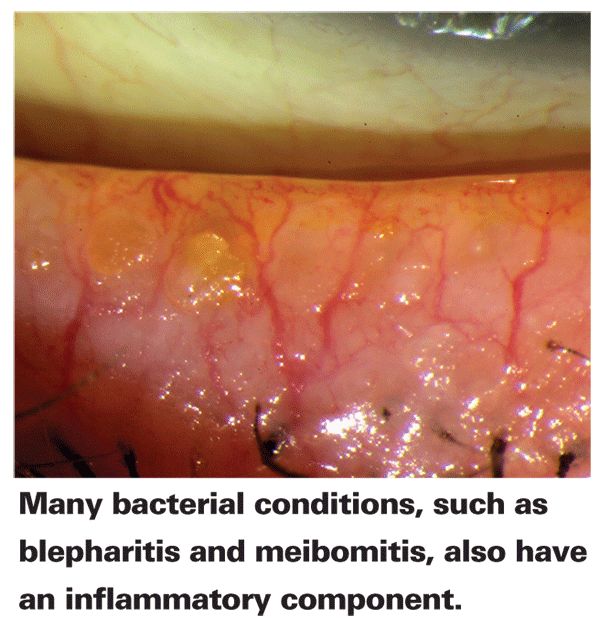
Other antibiotics of various classes have also exhibited anti-inflammatory properties. Currently, there have been too few studies to determine a trend for these agents. Nonetheless, some studies suggest these antibiotics can potentially be adopted as anti-infective and anti-inflammatory agents. Aminoglycosides, such as gentamicin and tobramycin, impair bacterial ribosomal function in a manner similar to that of tetracyclines. Gentamicin, discovered in 1963, inhibits inflammation through impediment of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, shown through studies with harvested human neutrophils.19 The NADPH oxidase exacerbates inflammation through production of various reactive oxygen species, which in turn activate the inflammatory pathway. By disrupting the NADPH oxidase activity, inflammation is prevented. Studies evaluating tobramycin in pigs have shown it exerts other anti-inflammatory properties, such as interleukin-6 cytokine inhibition.20 Furthermore, in a study evaluating various antibiotics in an intestinal inflammation model in rats, the broad-spectrum antibiotics imipenem and vancomycin in combination, administered via enemas, were found to mitigate inflammation.21
Clinical Relevance?
The causes of inflammation are not always known. Many conditions, such as sarcoidosis, are suspected to have bacterial involvement, but the exact nature of this involvement is uncertain. Other inflammatory conditions are known to have bacterial components, but eradicating the bacteria diminishes the inflammation, making it difficult to decipher a causal relationship. Because of this, clinical evaluations of the anti-inflammatory effects of antibiotics remain inconclusive. For example, the macrolide azithromycin has been suggested as an anti-inflammatory agent for cystic fibrosis, a condition related to abnormal bacterial levels in the lungs.22
In a clinical setting, tetracyclines modulate signs of inflammation. Patients with periodontitis treated with tetracycline over a 16-week period have shown marked improvement in clinical signs (i.e., bleeding and loss of connective tissue diagnosed by probing) with decreased levels of pro-inflammatory mediators.23 The same group, however, noticed a rebound in IL-1b secretion at 16 weeks, which may be undesirable from an anti-inflammatory agent.
There are few—if any—studies that have evaluated the ability of antibiotics to reduce non-bacterial inflammation definitively in comparison to steroids and NSAIDs. One study conducted in sarcoidosis patients, however, demonstrated the ability of antibiotics to reduce chronic inflammation that failed to respond to other treatments (hydroxychloroquine and prednisone). These patients were treated daily with minocycline over a 12-month period. Most patients showed remission of skin lesions during a follow-up period that lasted 26 months. In three out of the 12 cases of relapsing lesions following minocycline, patients were given doxycycline at the same dose, as doxycycline is known to have a minimal side-effect profile compared to minocycline, which resulted in complete remission of skin lesions.24
Similar findings of reduced inflammation were seen in studies involving different inflammatory diseases, including pemphigus, pemphigoid and rheumatoid arthritis.25-27 Although some of these diseases may be bacterial, studies in animals looking at inflammation that were referenced earlier suggest these antibiotics possess anti-inflammatory properties.
Studies in both animals and humans have shown that macrolides effectively reduce inflammatory mediators in a variety of diseases, including panbronchiolitis, cystic fibrosis and chronic sinusitis.15,23 One of the groups also found that a low dose of 500 ng/mL is sufficient to mitigate chronic obstructive pulmonary disease in humans.28
A case study suggested cotrimoxazole, a combination of the antibiotics sulfamethoxazole and trimethoprim, had anti-inflammatory properties when a patient with Wegener's granulomatosis showed improvement in symptoms and lower levels of pro-inflammatory mediators upon treatment with the combination.29 Finally, according to one research group, cotrimoxazole has a potential role for the treatment of rheumatoid arthritis as a result of its anti-inflammatory properties.30
Side Effects
While topical tetracycline preparations aren't currently available to the ophthalmologist, this class of agents represents a candidate for the treatment of a variety of ophthalmic diseases, and we may not have to wait much longer for these agents to be accessible. Despite being beneficial as dual-acting anti-inflammatories and anti-infectives, the tetracyclines aren't without flaw. Their efficacy compared to steroids is disputed: While doxycycline has been demonstrated to be superior to a steroid in reducing inflammatory mediators in a mouse model of dry eye, the results were not statistically significant.6 Also, oral minocycline treatment has been associated with some side effects, namely skin pigmentation changes following dermal treatment.25,26 Nonetheless, the value of these agents for treating inflammation with or without an infectious component can't be disputed.
A Rebirth
Although the extensive use of antibiotics has given rise to fears of emerging resistance, some of these anti-infectives may have a further purpose in the vast field of medicine. As this relates to ophthalmology, the future for treating ocular inflammation may not involve a steroid and fluoroquinolone combined in one bottle, but a single chemical entity that effectively blocks inflammation and kills infectious microbes when applied to the eye. The ability to match the potency of an anti-infective/anti-inflammatory agent with the strength of the condition will maximize recovery outcomes and minimize residual effects.
It should also be noted that that newer antibiotics will likely be engineered to have anti-inflammatory properties. But more important, the recent focus on the variety of beneficial effects provided by anti-infectives may provide a window into the future. The ideal therapy addresses multiple aspects of a disease with a single active agent, thus limiting the potential for adverse side effects and unanticipated drug interactions that exists with drug cocktails, and we expect a shift from the traditional "one sign or symptom, one drug" to "multiple signs or symptoms, one drug."
In the near future, we may have the option of treating with an anti-infective that blocks inflammation, an anti-inflammatory that also stimulates lacrimal gland secretion, an antihistamine that blocks itching, hyperemia and late-phase inflammation; and maybe even an intraocular pressure lowering agent that inhibits ganglion cell apoptosis. While we watch with great enthusiasm the development of antibiotics with anti-inflammatory capacity, we recognize that, for the clinician and in research studies, the anti-inflammatory efficacy of any new product will be benchmarked against steroids.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Duan K, Dammel C, Stein J, et al. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol 2003;50:5:1477-91.
2. Khan AA, Slifer TR, Araujo FG, Suzuki Y, Remington JS. Protection against Lipopolysaccharide-Induced Death by Fluoroquinolones. Antimicrob Agents Chemother 2000;44:11:3169-73.
3. Shapira L, Soskolne WA, Houri Y, Barak V, Halabi A, Stabholz A. Protection against Endotoxic Shock and Lipopolysaccharide-Induced Local Inflammation by Tetracycline: Correlation with Inhibition of Cytokine Secretion. 1996;64:3:825-8.
4. Suomalainen K, Sorsa T, Golub LM, Ramamurthy N, Lee HM, Uitto VJ, Saari H, Konttinen YT. Specificity of the anticollagenase action of tetracyclines: Relevance to their anti-inflammatory potential. Antimicrob Agents Chemother 1992;36:1:227-9.
5. De Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, Stern ME, Pflugfelder SC. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res 2006;83:526-35.
6. Krakauer T, Buckley M. Doxycycline Is Anti-Inflammatory and Inhibits Staphylococcal Exotoxin-Induced Cytokines and Chemokines. Antimicrob Agents Chemother 2003;47:11:3630-3.
7. Kloppenburg M, Dijkmans BAC, Verweij CL, Breedveld FC. Inflammatory and immunological parameters of disease activity in rheumatoid arthritis patients treated with minocycline. Immunopharmacology. 1996;31:163-9.
8. Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a Tetracycline Derivative, Is Neuroprotective against Excitotoxicity by Inhibiting Activation and Proliferation of Microglia. J Neurosci 2001;21:8:2580-8.
9. Kelly KJ, Sutton TA, Weathered N, Ray N, Caldwell EJ, Plotkin Z, Dagher PC. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 2004;287:760-6.
10. Lin S, Zhang Y, Dodel R, Farlow MR, Paul SM, Du Y. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett. 2001;315:61.
11. Amin AR, Attur MG, Thakker GD, Patel P, Vyas PR, Patel RN, Patel IR, Abramson SB. A novel mechanism of action of tetracyclines: Effects on nitric oxide synthases. Proc Natl Acad Sci
12. Sugiyama Y, Yanagisawa K, Tominaga SI, Kitamura S. Effects of long-term administration of erythromycin on cytokine production in rat alveolar macrophages. Eur Respir J 1999;14:113-6.
13. Baik AR, Lee J. Erythromycin Inhibits Interleukin-6 and Interleukin-8 Expression and Promotes Apoptosis of Activated Human Neutrophils In Vitro. Korean J Physiol Pharmacol 2007;11:259-62.
14. Miyanohara T, Ushikai M, Matsune S, Ueno K, Katahira S, Kurono Y. Effects of Clarithromycin on Cultured Human Nasal Epithelial Cells and Fibroblasts. Laryngoscope 2000;110:126-31.
15. Tkalcevic VI, Bosnjak B, Hrvacic B, Bosnar M, Marjanovic N, Ferencic Z, Situm K, Culic O, Parnham MJ, Erakovic V. Eur J Pharmacol. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. 2006;539:131-8.
16. Murphy BS, Sundareshan V, Cory TJ, Hayes D Jr, Anstead MI, Feola DJ. Azithromycin alters macrophage phenotype. J Antimicrob Chemother 2008;61:554-60.
17. Legssyer R, Huaux F, Lebacq J, Delos M, Marbaix E, Lebecque P, Lison D, Scholte BJ, Wallemacq P, Leal T. Azithromycin reduces spontaneous and induced inflammation in ¢F508 cystic fibrosis mice. Respiratory Research 2006;7:1:134.
18. Kohri K, Tamaoki J, Kondo M, Aoshiba K, Tagaya E, Nagai A. Macrolide antibiotics inhibit nitric oxide generation by rat pulmonary alveolar macrophages. Eur Respir J 2000;15:1:62-7.
19. Umeki S. Anti-inflammatory action of gentamycin through inhibitory effect on neutrophil NADPH oxidase activity. Comp Biochem Physiol 1995;110B:4:817-21.
20. Goscinski G, Lipcsey M, Eriksson M, Larsson A, Tano E, Sjolin J. Endotoxin neutralization and anti-inflammatory effects of tobramycin and ceftazidime in porcine endotoxin shock. Critical Care 2004;8:1:R35-41.
21. Videla S, Vilaseca J, Guarner F, Salas A, Treserra F, Crespo E, Antolin M, Malagelada JR. Role of intestinal microflora in chronic inflammation and ulceration of the rat colon. Gut 1994;35:1090-7.
22. Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:2:489-95.
23. Lamster IB,
24. Bachelez H, Senet P, Cadranel J, Kaoukhov A, Dubertret L. The Use of Tetracyclines for the Treatment of Sarcoidosis. Arch Dermatol 2001;137:69-73.
25. Ozog DM, Gogstetter DS, Scott G, Gaspari AA. Minocycline-Induced Hyperpigmentation in Patients With Pemphigus and Pemphigoid. Arch Dermatol 2000;136:1133-8.
26. Kloppenburg M, Dijkmans BAC, Verweij CL, Breedveld FC. Inflammatory and immunological parameters of disease activity in rheumatoid arthritis patients treated with minocycline. Immunopharmacology 1996;31:163-9.
27. Wise RD. Submicrobial doxycycline and rosacea. Compr Ther 2007;33:2:78-81.
28 Hodge S, Hodge G, Brozyna S, Jersmann H, Holmes M, Reynolds PN. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur Respir J 2006;28:486.
29. Ohtake T, Kobayashi S, Honjou Y, Shirai T, Takayanagi S, Tohyama K, Tokura Y, Kimura M. Generalized Wegener's Granulomatosis Responding to Sulfamethoxazole-trimethoprim Monotherapy. Intern Med 2001;40:7:666-70.
30. Rozin A, Schapira D, Braun-Moscovici Y, Nahir AM. Cotrimoxazole Treatment for Rheumatoid Arthritis. Sem Arthritis Rheum 2001;31:2:133-41.











