Andover, Mass.
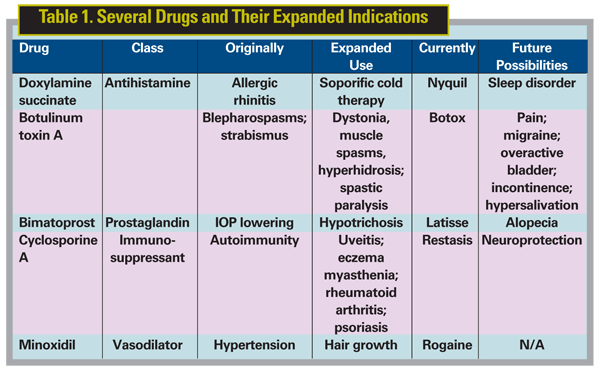
Just as discoveries at the laboratory bench can be translated into useful clinical applications, previously commissioned drugs can be translated into novel treatments for other unrelated conditions. Viagra (Sildenafil 2.5%, Pfizer), for example, has demonstrated cardio-protective effects subsequent to its use as a hypertensive and erectile dysfunction medication.1 Similarly, NyQuil (Procter & Gamble) was originally marketed as an antihistamine, but its potent blocking of central H1 receptors produced a sedative effect; it's now used to treat cold symptoms, where the soporific result is much-appreciated. When a drug's mechanism of action suggests cross-application—or, when a medication becomes prohibitively expensive for some patients—doctors sometimes prescribe medications for off-label use. While the courts protect the doctor's right to prescribe off-label, recent restrictions and legislation have made doctors less willing to make off-label recommendations.
Many ocular medications originate from prior systemic compounds that are then simply re-researched with regard to safety and efficacy for use in the eye. These converted formulations span all areas of ophthalmics: dry eye, allergy, anti-infection, inflammation and auto-immune disorders. Understanding their mechanisms of action can aid physicians in evaluating off-label uses for existing drugs, a process that may eventually lead to approval of the extended indication.
From Pharmaceutical to Cosmeceutical
Drug activity is not linear. Often it's a process of accidental discovery. A classic example of this is penicillin, which was discovered as a moldy ring on a blood agar plate that exhibited bactericidal properties. A host of products have been developed to improve patients' physical characteristics based on the unexpected side effects of more medically oriented medications. Botulinum toxin was isolated from mold spores discovered in meat products in the mid 1800s.2 Alan Scott, MD, of the Smith-Kettlewell Institute was the first to test it for treatment of strabismus,3 and it was later developed into a drug to be injected into the muscles surrounding the ocular orbit to manage blepharospasms and strabismus. This treatment interrupts neuromuscular transmissions, thereby reducing spasms.4 But in anything but minute quantities, it remains a deadly toxin.
Impeding these muscle hyperactivities may have been the original indication for botulinum toxin, but it has been introduced into many new arenas since its discovery in 1895. Beginning with the observation that botulinum injections provided temporary relief from tension and migraine headaches, different strains have been isolated to treat more than a dozen indications. These include the popular Botox (Allergan) injections to diminish glabellar lines (frown lines between the eyebrows) as well as potentially to treat abnormal perspiration as well as gastrointestinal conditions such as obesity,5 overactive bladder and hypersalivation.6
Perhaps one of the best-known translations of a pharmaceutical into a cosmetic therapy is the vasodilator minoxidil, which is useful not only as a systemic hypertension medication but also as the active ingredient in Johnson & Johnson's Rogaine.
Researchers explored its hair-growth properties after observing the increasingly hirsute appearance of subjects with high blood pressure participating in clinical trials of minoxidil.7 Hair regrowth became and remains its primary indication.
Thirty years ago, prostaglandins were known as the probable culprit in the production of hypotony in association with uveitis. Using the adverse effect of an inflammatory mediator as a therapy was unimaginable until Laszlo Bito, PhD, of Columbia University recognized and developed it.8 Prostaglandin analogues have been synthesized since the 1990s and lower intraocular pressure through increasing the outflow of aqueous humor through the trabecular meshwork. Prostamides, such as bimatoprost, exhibit very few systemic side effects, as their effects are concentrated in the eye.
Ophthalmic side effects of prostaglandin analogues included the usual conjunctival hyperemia and ocular irritation that occur frequently with the use of the agents, but unusual side effects also manifested. Notable eyelash lengthening and thickening was perceived by many patients. Bimatoprost causes changes in iris, eyelash and eyelid pigmentation as well as lash lengthening and thickening.9 This hypertrichosis may be mediated by vasodilation. Latanoprost, another prostaglandin analogue, induces the anagen (growth) phase in telogen (resting) hair follicles to bring about hypertrophic changes.10
Bimatoprost has been prescribed off-label for cosmetic purposes for some time. However, it wasn't until late in December of 2008 that Latisse (bimataprost 0.03%, Allergan) became FDA-approved for treating hypotrichosis: stunted eyelash growth. Side effects of this new product include eye pruritis, conjunctival hyperemia and eyelid hyperpigmentation in 3 to 4 percent of users, but the iris hyperpigmentation seen with Lumigan application is no longer a factor.11,12 Due to the balance of safety and efficacy, this will likely become a widely used product.
Undoubtedly, we will continue to learn from newly adapted medications. Another side effect of prostaglandins is inflammation manifested as hyperemia. With this in mind, maybe an anti-inflammatory, applied similarly to the Latisse "eye liner" applicator, could inhibit lash growth. This would be useful in cases of pemphigoid, the only disease where increased eyelash growth is associated with inflammation. Perhaps an anti-inflammatory could decrease unwanted lash growth, or perhaps not.
Treating Multiple Ailments
The process of inflammation is necessary for wound healing; however, it is often undesirable and the subject of pharmacological modulation. The non-ribosomal peptide known as cyclosporine, discovered in 1972,13 inhibits signal transduction pathways through binding to the immunophilin calcineurin. It was discovered as a result of a screening program for antibiotics with immunosuppressive activity and minimal cytotoxicity. A fungal extract was submitted to the program in the early 1970s and cyclosporine was identified as its main component.14 As originally approved, cyclosporine was used as an immunosuppressant to prevent transplant rejections, but it has since expanded into many medical spheres, including psoriasis, rheumatoid arthritis, and Crohn's disease. Chronic dry eye (keratoconjunctivitis sicca) can result in a cycle whereby symptoms accompany inflammation in some dry-eye patients. Left untreated, chronic dry eye can result in damage to the ocular surface, decreasing visual acuity. Cyclosporine, acting as an immunomodulator with anti-inflammatory effects, dramatically increases natural tear production in approximately 15 percent of dry-eye patients.15
A Modest Proposal
All in the medical profession sympathize for the sufferer of a disease and wish to ease his pain. Those with more common ailments may find reprieve in the multitude of remedies available without prescription or in generic form, but refuge is more difficult to attain for the victim of an orphan disease. The rarer the malady, the tougher the road to recovery—and to drug development—as development costs likely exceed any profits that may be made on such a seldom-used therapy. A demonstrated need must be evident for the compulsory inquiry to commence. This is a complex and protracted endeavor that readily consumes time and finance, and increasingly defies expectations. The probability that it will end in favor of a treatment is slim, and the risk for the researcher severe. However, the reward for the investigator is a blend of achievement, fulfillment and compensation.
Although intrinsic recompense is satisfying, extrinsic return is vital to the wellbeing of the creator, as well as the maintenance of the industry. Philanthropy is part of societal duty, but it cannot be the mainstay of a venture. If such were to be the case, the burden would simply be transferred elsewhere; as the fixed overhead would still require disbursement, the brunt would then have to be borne by other patrons, larger philanthropists or public funds. It would be a disservice to many more to increase the cost of conventional therapies, and the populace able to contribute to such a cause is not only finite but likely to be already allocated elsewhere. Public capital, too, is stretched thin, though it is allotted to research focusing on the most severe and widespread afflictions. Sufferers are invited to participate in the very trials endeavoring to alleviate their disorders, and are thus contributing to their cure as well as being able to earn compensation for their effort.
An example of this controversy is the case of Lucentis (ranibizumab, Genentech), the ophthalmic formulation of the systemic precursor Avastin (bevacizumab, Genentech), used to treat metastatic colon cancer. Judah Folkman, MD, of Harvard Medical School16 published a seminal paper purporting that angiogenesis inhibition could be used to hinder tumor growth. Chiefly due to this insight, angiogenesis inhibitors have become a mainstay in cancer and wet age-related macular degeneration treatment, with Lucentis as the current front-runner in wet AMD treatment.
In 2005, the cost of Lucentis injections was about $2,300 each; they are recommended once a month for two years, per the results of the
Comparatively, Avastin, derived from exactly the same monoclonal antibody, costs about $50 per treatment. Due to its off-label nature, it doesn't come pre-loaded in a syringe and therefore carries extra risk and less convenience. Also, it is not approved or manufactured for ophthalmic use, and as long-term ophthalmic data are not available for Avastin, the Lucentis safety and efficacy profiles may be stronger. Because it is not FDA-approved for the treatment of wet AMD, Avastin prescriptions are done off-label.
Despite the legality of off-label use, it has many ethical and moral concerns. But through off-label use, as well as the many safety trials for systemic Avastin, Avastin has been demonstrated to be safe and efficacious for the treatment of wet AMD, although this "anecdotal" evidence cannot substitute for randomized clinical trials.
Ethical considerations to be taken with off-label prescription include recompense for adverse side effects and proprietary infringement. A non-industry, direct comparison study by the National Institutes of Health could lay to rest the debate over superior safety and efficacy, and the explicit approval for it by the FDA expressly for wet AMD could cease off-label prescription of Avastin.
Deciding how to fairly price prescription medications has been left unsettled, and the question of whether the government should intervene is also unresolved. One reason for this is a great equalizer inherent in the system: patent expiration. Gross windfall profits shouldn't occur on a regular basis, but a new drug that dramatically affects people's quality of life should result in a high payout. However, it's only a matter of time until the drug goes off patent and the creator no longer nets as large a profit from it. Reward is necessary for the capital to re-invest in further, risky research. Also, if it's a genetic disease, the cost will go toward developing next-generation drugs for the next generation of patients. These aspects of drug development must also be considered when evaluating the relatively high costs of some drugs.
Clearly, the approval of a newly discovered compound for clinical use is but the first step in the drug development process. Through widespread use, approved drugs are constantly evaluated for potential side effects, and their safety monitored. At that point they become prime targets for further translation. A medication's side effects, or newly discovered activities sussed out by the intuitive clinician, can permit its therapeutic benefits to be expanded, often to other regions of the body. Since these agents have already been proven safe, either systemically or in a particular organ, it's often faster and easier to get additional FDA-approved indications. This should be the ultimate goal of successful, off-label usage: to lead to a full clinical development program. Only with a full program can the range of safety and efficacy, drug interactions and responder/non-responder groups be properly identified for the benefit of patients and the protection of the prescriber.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart and Circ Physiol 2002; 283:3:H1263-9.
2. Erbguth FJ. From poison to remedy: the chequered history of botulinum toxin. J Neural Trans 2008;115:4:559-65.
3. Dutton JJ and Fowler AM. Botulinum toxin in ophthalmology. Survey Ophthal 2007;52:1:13-31.
4. Gilman AG, Rall TW,
5. Carruthers A. Botulinum toxin type A: history and current cosmetic use in the upper face. Dis Mon 2002;48:299-322.
6. Search criteria: Botox. Retrieved January 2, 2009 from Informa Healthcare Pipeline Website: http://ora-pipeline.citeline.com/CpResultDrugs.aspx?UserSearchID=29648 &Refine=0.
7. Kolata G. Hair-growth drug approved, the first cleared in the
8. Yorio T and Dibas A. New therapies for glaucoma: Are they all up to the task? Ex Op on Ther Pat 2004;14:12:1743.
9. Cracknell K and Grierson I. Prostaglandin analogues in the anterior eye: their pressure lowering action and side effects. Exp Eye Res 2008:1-6.
10. Johnstone MA and Albert DM. Prostaglandin-induced hair growth. Survey of Ophthalmology 2002;47:1:S185-202.
11. Allergan Prescribing Information for Lumigan 2006
12. Allergan Prescribing Information for Latisse 2008
13. Drews, J. Drug discovery: A historical perspective. Science 2000;287:1960.
14. Heusler K, Pletscher A. The controversial early history of cyclosporine. Swiss Med Wkly 2001;131:299-302.
15. Allergan Prescribing Information for Restasis 2008
16. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:12:1182-6.
17. Steinbrook R. The price of sight – ranibizumab, bevacizumab, and the treatment of macular degeneration. New Eng J Med 2006;355:14:1409-12.
18. Current population survey 2007. Retrieved January 13, 2009 from US Census Bureau Website: http://www.census.gov/Press-Release/www/releases/archives/income_wealth/ 010583.html.











