Endothelial keratoplasty has evolved dramatically in recent years, and it continues to do so. Each new iteration promises less trauma for the diseased eye and graft tissue, along with better visual outcomes; yet each advance is also sufficiently complex that it requires years of refinement before achieving widespread use. Here, several experts in current and upcoming variations on endothelial keratoplasty—especially Descemet's Stripping Endothelial Keratoplasty and its partially automated variation, DSAEK—share their latest insights, experience and thoughts about what lies ahead.
Managing the Donor Tissue
One of the biggest challenges in DSEK and DSAEK is minimizing en--
dothelial cell loss during the handling and insertion of the donor tissue into the recipient eye. In general, the greater the endothelial cell loss, the greater the likelihood of a poor outcome. Recent research suggests that key factors in reducing endothelial cell loss include how the tissue is handled, the location of the insertion wound, the size of the wound and use of an insertion device.
"We've been doing DSEK for more than five years, and we have the longest follow-up of anybody in the country," notes Francis W. Price Jr., MD, president of the Price Vision Group in
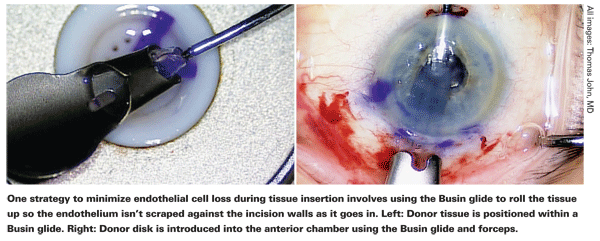
"We also discovered early on that there was less damage if we used a clear corneal incision instead of a scleral tunnel," he continues. "That probably has to do with the length of transit of the tissue through the incision and the fact that in the scleral tunnel you have more change in curvature between the sclera and the cornea. However, a scleral tunnel is self-sealing, requires no sutures, and you don't get the astigmatic shift that you do with a clear corneal incision." Dr. Price notes that using a Busin glide with a scleral tunnel helps to protect the tissue. "In fact, we don't find a correlation between incision location and cell loss if we use a Busin glide," he says. "The glide rolls the tissue up so the endothelium isn't scraped against the incision walls as it goes in."
Mark A. Terry, MD, director of corneal services at the Devers Eye Institute in
"What we found was quite startling," he continues. "With the same surgeon, it didn't matter which technique you used to put the tissue inside the eye. The overriding factor in endothelial cell death was the size of the incision. If you used a 3-mm incision, it was much, much worse than using a 5-mm incision—with a p-value of .001. If you think about it, this is perfectly logical. If you put an 8-mm tissue through a 5-mm incision, it gets compressed, but if you put it through a 3-mm incision, it really gets compressed. This result was uniform across every technique we tested.
"The main difference we found in terms of technique alone was that folding the tissue actually caused less cell damage," he adds. "Not folding the donor graft was associated with the death of 50 to 60 percent of endothelial cells—even through a 5-mm wound. Other differences between the techniques were not significant in terms of cell loss as long as the 5-mm incision was used."
Of course, an injector system could in theory avoid many of these concerns. "Injectors have the potential to be beneficial because they should minimize the compression damage that occurs as the tissue goes through the incision," explains Dr. Price, noting that his group has tried several different systems. "In addition, some don't require making an extra incision to pull the tissue into the eye. However, they use different principles to roll up or curl the tissue, and we don't know how much compression damage occurs during that process. There are other concerns as well: How easily does the tissue disengage? Does the process create folds or wrinkles in the tissue? It will be six months to a year before we have data on these questions from multiple sources that aren't involved in the development of a particular injector."
Keeping the Graft in Place
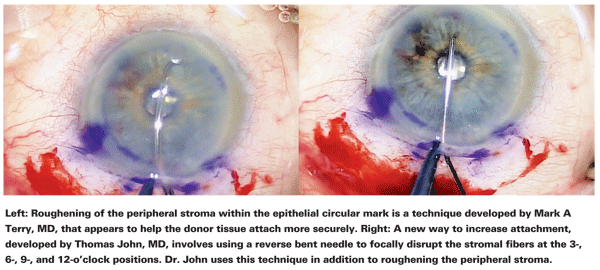
Another major concern with any form of endothelial keratoplasty is preventing graft slippage once the donor tissue is positioned inside the eye. Dr. Terry has published several articles detailing the low graft dislocation rate his group has produced—1.5 percent in their most recent series.2,3 Dr. Terry believes the main factor that accounts for his group's low dislocation rate is the scraping of the periphery of the recipient bed.
"We learned from comparing the histology of DLEK and DSEK that when you have a recipient bed with a lot of cut stromal fibers, those cut fibers dramatically increase the surface area of the recipient bed—yet they keep the diameter of the recipient bed the same," he explains. "Any time you have a larger surface area, you get greater adhesion.
Also, when you push the two surfaces together with cut fibers on both the donor and recipient tissues, some of those fibers are going to entwine around each other and act like little bits of Velcro.
"After we strip Descemet's membrane, which usually leaves us with an 8- or 9-mm circle, we scrape about a 1-mm wide ring in the periphery of that recipient bed," he continues. "To do the scraping, we use a specially designed scraper that's 1 mm wide. It's reminiscent of an ice scraper—it's not subtle. You need to scrape the tissue aggressively so that you're left with a visible ring of light fibers. I've had surgeons say to me, 'I do the scraping, but I still have a high dislocation rate.' It usually turns out that they're scraping with a blunt Sinskey hook, or forceps, or a stripper with a blunt tip. I tell them they're not doing enough scraping."
Dr. Price believes wound construction is more important. "In my experience, the most common reason for detachments is a slow wound leak," he says. "The eye gets soft, and when the patient blinks the cornea is indented, triggering detachment." Dr. Price doesn't believe the technique of roughing up the edge of the graft to aid attachment makes a significant difference. "I know some surgeons favor that approach, but our results are pretty much identical, at least in terms of detachment rate.
"Also," he continues, "we primarily use surgeon-cut tissue rather than pre-cut tissue. With pre-cut tissue there are some issues involving the Dextran in which the tissue is stored. Dextran is kind of slippery, which may undermine attachment, and some surgeons have suggested that Dextran can diffuse into the stroma after it's been cut and stored for a while, causing the donor tissue to swell after it's put into the eye, which can also lead to detachments. Using surgeon-cut tissue, we haven't found detachment to be a big issue with DSEK for the past three or four years."
Thomas John, MD, clinical associate professor at
"My third technique, which is de- scribed in detail in an article that will be published shortly in Ocular Surgery News, involves using a re- verse bent needle to focally disrupt the stroma fibers at four areas—3-, 6-, 9- and 12-o'clock," he adds. "I haven't had any detachments since I began using this three-pronged approach."
No matter what protocol surgeons follow to prevent graft dislocations, everyone agrees that the rate will never go to zero, because patient be-havior postop is out of the surgeon's control. "If your patient has blepharospasm, or rubs her eyes, or squeezes hard when she puts in drops, she's more likely to have a dislocation," Dr. Price notes. "Except for those patients, you really shouldn't see dislocations."
Removing Interface Fluid
Fluid that's trapped between the recipient surface and the donor graft can be a major contributor to graft dislocation. Several methods—some controversial—can be used to eliminate fluid without disturbing the graft.
"Decreasing the workload of the donor endothelium helps it adhere," says Dr. Terry. "One way to do that is to eliminate as much interface fluid as possible at the time of surgery. We do that by surface sweeping. We put the tissue in place and fill the chamber with air to create elevated pressure, anywhere between 35 and 65 mmHg. With that pressure pushing the graft up into place, we irrigate the corneal surface and use a device such as a Cindy Sweeper or a Lindstrom LASIK roller to compress the cornea from the center to the periphery, past the edge of the graft, for 30 to 60 seconds. Any fluid is squeezed out and drops into the anterior chamber.
"There are several ways to determine whether all the fluid is gone," he continues. "For one thing, after 15 or 30 seconds of sweeping, you'll see that the graft tissue is no longer moving. Also, once you finish sweeping you can look at the cornea using very high microscope magnification. Because the donor tissue always has some degree of edema, you can see a specular microscopy image of endothelium on the side of a donor fold. If you can see hexagonal cells, there's no interface fluid at that location; you can't see that level of detail through fluid. If there is fluid present, you'll see a lake; it will look crystal clear and smooth, with no folds or endothelium. That's a bad sign.
"Another sign is that there may be small air bubbles trapped in the interface from the insertion process," he continues. "If those air bubbles move as you sweep from the center to the periphery, that means they're floating in fluid that still remains. If there's no fluid there, those air bubbles are trapped in the stroma and don't move at all. That's what you want to see." Dr. Terry notes that tiny air bubbles left in the interface are absorbed within an hour or two.
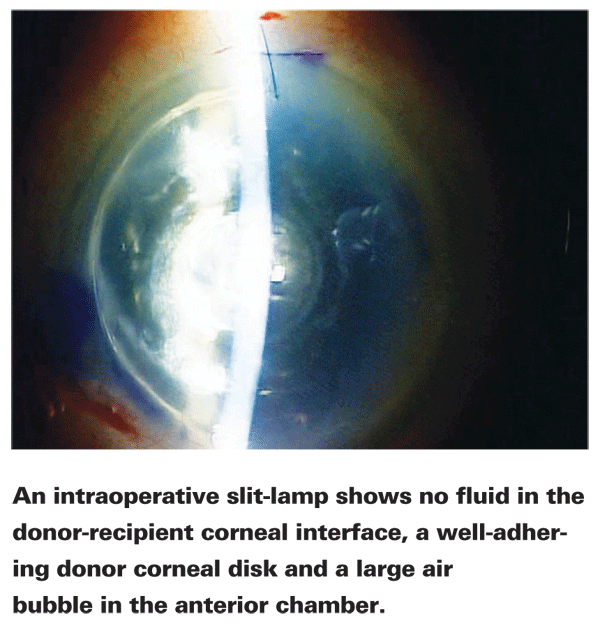
Dr. John uses a slit lamp attached to his microscope in the OR to determine whether or not fluid needs to be removed from the interface. (See image, above.) "With the slit beam, you can sweep the cornea from limbus to limbus; if there's any fluid, you'll see the donor disc being pushed away from the cornea. Many operating room microscopes don't have a slit lamp attachment, but several manufacturers do offer them. The slit lamp is very beneficial; you can actually see which level of the cornea you're at during dissections."
Dr. John says he removes any fluid using either a roller or increased air pressure in the anterior chamber. "If you sweep the dome of the cornea you have to be gentle," he points out. "If you're too vigorous, you may move the disc from its seated position. In fact, that technique is sometimes used to reposition a misaligned disc. Alternatively, I increase the amount of air in the anterior chamber; the air pushes on the donor disc, expelling any fluid from the interface. However, if you do this, at the end of the case you have to make sure you've decreased the air bubble size and made a peripheral iridectomy, either mechanically or using an Nd-YAG laser, to decrease the likelihood of a pupillary block."
Dr. Price says that he uses drainage incisions (stab incisions into the cornea) in most of his DSEK cases to remove fluid trapped in the interface. "I used to use four stab incisions, but now I typically start out with one on the temporal side to see if there's any fluid," he explains. "You get a little swelling from the wound there, which pushes the donor tissue away a little bit. If you don't get any fluid making an incision there, you're not going to get it in the other areas either." Dr. Price adds that a common mistake is making radial incisions for this purpose. "You want to make the incisions parallel to the limbus and about 1 mm in from the edge of the graft," he says. "Radial incisions are more likely to extend to the edge of the graft and beyond. That can lead to a wound leak and a possible detachment."
Dr. Terry believes the incisional approach to fluid draining has too many potential downsides. "Stab incisions create a liability for the eye postoperatively," he says.
"We've seen several reports of this leading to epithelial downgrowth from the surface into the interface, resulting in interface opacities. And that's not as bad as several reports of bacteria and fungi getting into the interface through drainage stab incisions. Why put the patient at any risk when you don't need to? You can simply sweep the interface fluid out and then you're done."
Dr. Price says his experience doesn't support these concerns. "We've done more than a thousand cases using stab incisions to drain fluid, and I only had one epithelial ingrowth a while back," he says. "I know that some surgeons have reported a problem with this, but I believe it's almost uniformly been in cases in which they weren't just using the incisions for drainage; they were also manipulating the donor tissue through those incisions, which could drag epithelium into the wound. Incisions like that are not designed for manipulating tissue."
Preventing Pupillary Block
One concern associated with leaving an air bubble inside the eye postop to help hold the graft in place is the possibility of triggering pupillary block glaucoma. "Pupillary block glaucoma can be a disaster for the eye," says Dr. Terry. "If you leave enough air in the anterior chamber to occlude the pupil and cause the postop pressure to rise to 60, 70 or 80 mmHg overnight, the patient can get a permanently dilated pupil and can also permanently lose vision. Many surgeons are taught to fill the chamber with air, check the patient an hour later, burp some air out and hope for the best, but most surgeons who use that technique report at least one or two cases of pu- pillary block. Some use an inferior peripheral iridectomy to offset this, so if the patient sits up fluid can come out from behind the iris and fill the chamber. To me, that's fixing a problem that you didn't need to create.
"Here's our protocol," he continues. "After we've done our sweeping and the tissue is locked into place with no interface fluid, we let the tissue sit for 10 minutes undisturbed with the air still in the chamber at a normalized pressure. At the end of the case, we turn the patient's head away from us (i.e., the eye is facing nasally) so the temporal paracentesis site is at the top of the bubble. We put BSS in through the paracentesis using a cannula; that causes the air to come up and escape from the eye, replaced by the BSS. This takes five seconds or less. We leave a 2- or 3-mm free-floating air bubble in the anterior chamber because we don't want to touch the endothelium trying to get the last little bit of air out.
"We then turn the patient's head back into position so he's facing the ceiling," he says. "We make sure that the chamber is deep, the iris is flat and the pupil, which was dilated during the 10-minute waiting time, is larger than the air bubble. The tissue stays in place because it's well-attached. In fact, it probably doesn't need an air bubble for support at all because the fibers we created by scraping the periphery of the recipient bed are holding it in place. There's no interface fluid. And if we've been gentle with the tissue and protected the en-dothelium, then some of the endothelial cells are starting to wake up and pump, helping adherence."
Dr. Terry says that the air bubble is not essential for adherence at the end of surgery. "We may inject a little air to leave a 4- or 5-mm bubble, just to provide a little bit of central support for the graft," he notes. "The periphery doesn't seem to need the support. But we don't use an air bubble that's as large as the graft. And we certainly don't leave an air bubble that's larger than the pupil or attached to the pupil. The bubble has to be freely floating. If you follow these steps, you'll avoid pupillary block."
DMEK and DMAEK
The point of any of these surgeries, of course, is to replace malfunctioning endothelial tissue. Other layers of cornea that are transplanted along with the endothelium facilitate the transplantation process, but ideally—at least in theory—those layers do not need to be part of the graft at all. The latest iteration of the process, Descemet's Membrane Endothelial Keratoplasty, moves it in that direction by transplanting only Descemet's membrane and endothelium—no stroma.
"DMEK surgery represents pure anatomic replacement of the pathology," notes Dr. Terry. "You take out the diseased Descemet's membrane and put in a healthy one. Unfortunately, the devil is in the details. So far there are only a handful of published articles about DMEK, and all of those are by one surgeon, Gerrit Melles in the Netherlands, discussing the same series of patients.4

"The good news," he continues, "is that DMEK appears to produce visual results about one line better than what we're getting with DSAEK. In my series of hundreds of DSAEKs, about 13 percent reached 20/20; in his DMEK series, 20 to 25 percent of patients did. Similarly, 35 to 40 percent in our DSAEK series reached 20/25 or better; he had 60 to 70 percent in his smaller DMEK series. However, in his first 50 cases Dr. Melles also had a 25-percent dislocation rate and a 20-percent primary graft failure rate. Even a 3-percent primary graft failure rate would overwhelm the eye bank system."
"Ideally, DMEK is a welcome concept," observes Dr. John. "You avoid the problem of the disc causing a refractive shift. And if the disc is a little larger than the recipient area, it shouldn't cause a problem, which is not necessarily true in DSEK and DSAEK.
However, the surgical technique is very demanding. The endothelium, once detached from the donor cornea, has a tendency to curl up with the endothelial cells on the outer side. Handling the tissue is difficult. And once it's attached to the cornea, you don't have the luxury of moving the disc with a Sinskey hook as you might with a DSEK disc, because you can tear the membrane. However, like any surgical technique, it's evolving. Ultimately, I believe we will overcome these hurdles, and DMEK may turn out to be superior to DSEK."
Dr. Price's group has already done more than 100 DMEK-style procedures. "We're getting a one- to two-line improvement over DSEK," he says. "More than half of the DMEK patients are 20/20 or 20/25, and they're achieving good vision really quickly."
Dr. Price explains that his group is already working on a new variation of this procedure which they call DMAEK. "DMAEK is a hybrid of DMEK and DSEK in which we use the microkeratome to do the resection on an artificial anterior chamber," he says. "After we remove the donor tissue from the chamber, we flip it over so the endothelial side is up and inject air into the stroma, creating a bubble, but just in the center of the cornea. Next, we coat the tissue with viscoelastic and mount it on the artificial anterior chamber again; then we cut off the stroma over the big bubble. Finally, we dismount it again, put it on a cutting block and punch it. What we're left with is a graft with just Descemet's membrane and endothelium in the center and a rim with the stroma still attached.
"The big advantage of this approach is that when we put the tissue into the eye, the tissue unfolds immediately because of that outer doughnut of stroma," he continues. "With DMEK, you can have problems unfolding the tissue and moving it into place. This alternate method avoids those problems, and patients are getting phenomenal vision within a week or two of the surgery."
Dr. Price admits that there are still problems to address. "The downside of both DMEK and DMAEK is that they often require re-injections of air," he says. "The tissue is more finicky and doesn't stick as well. So we re-inject air a few days postop in at least half of these patients to help the tissue adhere. In terms of cell counts, we don't have a lot of data yet, but early returns are looking very good. Graft rejection is still possible, but it will take a few years to find out what the rejection rate is."
Dr. Price believes the technical problems with DMEK and DMAEK will be resolved over time. "This has been the case for most procedures," he notes. "I believe eventually we'll be using DMEK or DMAEK for the majority of our patients."
What's on the Horizon?
"The ultimate solution will be to take the recipient's endothelium, amplify it in the laboratory and then put it back inside the same eye we took it from," says Dr. Terry.
"Beautiful work is being done in Japan on amplification of endothelial cells; they've gotten it to work in animal eyes. So I hope that we're less than a decade away from that goal. If we achieve that, we'll have a perfect operation—a graft that is consistently successful, produces excellent vision quickly and will never be rejected."
1. Terry MA, Saad HA, et al. Endothelial Keratoplasty: The influence of insertion techniques and incision size on donor endothelial survival. Cornea 2009;28:24-31.
2. Terry MA, Shamie N, Chen ES, Hoar KL, Friend DF. Endothelial Keratoplasty: A simplified technique to minimize graft dislocation, iatrogenic graft failure and pupillary block. Ophthalmology 2008;115:1179-1186.
3. Terry MA, Shamie N, et al. Endothelial keratoplasty for Fuchs' dystrophy with cataract: Complications and clinical results with the new Triple Procedure. Ophthalmology 2009;116:631-9.
4. Ham L, Dapena I, van Luijk C, van der Wees J, Melles GRJ. Descemet's membrane endothelial keratoplasty (DMEK) for Fuchs' endothelial dystrophy: review of the first 50 consecutive cases. (EYE, advance online publication, Jan 2009).











