|
Advertisement

|
 |
|
Editor's Notebook |
And the Survey Says...
The responses to polls on the U.S. Pharmacist and PharmQD Web sites are often surprising. |
 |
|
Counseling Pearls |
 Combination Therapy Options in Stable COPD
Combination Therapy Options in Stable COPD
Chronic obstructive pulmonary disease is projected to become the third leading cause of death in the world. The concurrent use of beta agonists with muscarinic antagonists and/or corticosteroids plays a role in reducing the burden of this disorder.
|
 Post-Stroke Aspiration Pneumonia
Post-Stroke Aspiration Pneumonia
Seniors, especially stroke patients, are at an increased risk for developing serious comorbidities such as aspiration pneumonia, a lung infection caused by inhaling mouth secretions, stomach contents, or both. |
 |
|
It's the Law |
Electronic Controlled Substances Prescriptions
New federal regulations allow controlled substances to be prescribed electronically, which should help prevent errors and decrease diversion. |
| |
|
Clinical Corner |
 Nosocomial Pneumonia
Nosocomial Pneumonia
Hospital-acquired infections have a profound impact on the health care system and are an important cause of morbidity and mortality despite preventive measures and improvements in technology and antibiotic therapy. |
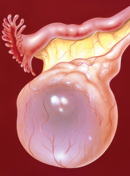 Ovarian Cysts: A Review
Ovarian Cysts: A Review
Ovarian cysts are a common cause of surgical procedures and hospitalizations among women worldwide. Thus, it is important for pharmacists to be knowledgeable about treatment options and the risk of malignancy. |

 Cleansing and Detoxification Products in Self-Care Cleansing and Detoxification Products in Self-Care
Patients should be advised that OTC detoxifying agents have not been approved by the FDA and should be used with caution. Products are available in a variety of delivery forms, including topical patches, liquids, enemas, and capsules. |
| |
|
Consult Your Pharmacist |
Preventing and Treating Summer Sports Injuries
It is important for pharmacists to be able to assess whether an injury is self-treatable or if a referral is required. |
 |
|
Educational Spotlight |
Hyperthyroidism: Exploring Treatment Strategies
It is important for pharmacists to understand and be able to educate patients about this endocrine disorder and the methods used to treat it. |
Optimizing Disease Control to Manage RA-Associated Pain in Adults
Patients with rheumatoid arthritis benefit greatly from coordinated, collaborative care that includes therapies designed to provide optimal pain control. |
| |
|
 Carry the US Pharmacist's Platinum Plus® MasterCard credit card!
Carry the US Pharmacist's Platinum Plus® MasterCard credit card!
Carry the only card that helps support our programs and initiatives at no additional cost to you. Simply
click here to apply online today. |
|
 |
| Newswire |
 |
 |
Avandia to Remain on the Market
Gaithersburg, MD — An FDA advisory panel has voted to keep Avandia (rosiglitazone) on the market despite concerns over increased heart attacks among patients. The type 2 diabetes drug is manufactured by GlaxoSmithKline (GSK) and has included a black box warning regarding heart failure since 2007. This ruling will require additional warnings to be added to the drug's labeling. The panel also voted to recommend continuation of the TIDE trial, which compares GSK's Avandia with Takeda Pharmaceutical's Actos (pioglitazone). In addition, GSK has agreed to pay $460 million to patients to settle a class action suit over Avandia. "Patients taking Avandia should speak with their physician about their treatment and any questions they may have regarding the safety of the medicine," Dr. Ellen Strahlman, GSK's Chief Medical Officer, said in a statement. |
 |
 |
Revised Standards for Electronic Health Records
Washington, DC — The Department of Health and Human Services (HHS) has issued new rules that will reward physicians and hospitals for the "meaningful use" of electronic health records. Over the next 10 years, health care providers could receive up to $27 billion to computerize medical records. The health care industry has been slow to replace paper records. "Only 20% of doctors and 10% of hospitals use even basic electronic health records," said HHS Secretary Kathleen Sebelius. The new rules are less demanding and significantly reduce proposed requirements that the health care industry had denounced as unrealistic. For example, physicians will be required to transmit 40% of prescriptions electronically, instead of a proposed 75%, to meet the new standards. Starting in 2015, there will be financial penalties under Medicare for not using electronic health records. |
 |
 |
Weight Loss Drugs Under FDA Review
Gaithersburg, MD — Due to safety concerns, an FDA advisory committee has voted against approval of Vivus Inc.'s anti-obesity drug Qnexa (phentermine/topiramate). The panel cited the need for more long-term safety data and expressed concern over reports of teratogenicity, depression, and memory problems. The drug includes two previously approved components, phentermine, an appetite suppressant that was once part of the recalled fen-phen, and topiramate, an anticonvulsant that promotes satiety. In several studies, those who took high-dose Qnexa lost about 10% of their body weight after 1 year. Two other potential weight-loss treatments, lorcaserin (Arena Pharmaceuticals) and Contrave (Orexigen Therapeutics), will also undergo FDA review later this year. |
 |
|
