A 65-year-old Hispanic male presented with decreased vision in his right eye. He reported a dramatic change in his vision about one month earlier. He also noted mild ocular discomfort in the same eye.
A 65-year-old Hispanic male presented with decreased vision in his right eye. He reported a dramatic change in his vision about one month earlier. He also noted mild ocular discomfort in the same eye.
He had no significant ocular history, but his medical history was significant for hypertension, which he controlled with catopril.
On examination, best-corrected visual acuity measured 8/200 O.D. and 20/20 O.S. Confrontation visual fields were grossly constricted in the right eye, but were full to careful finger counting in the left eye.
Pupils were equally round in both eyes. The left eye was briskly reactive to light. The right eye, however, was very sluggish, with a strong afferent pupillary defect.
 The anterior segment O.D. was significant for a bowing forward of the iris, with narrowing of the anterior chamber (AC) both centrally and peripherally; the left eye was 3+ deep without cell or flare. No iris rubeosis was visible in either eye.
The anterior segment O.D. was significant for a bowing forward of the iris, with narrowing of the anterior chamber (AC) both centrally and peripherally; the left eye was 3+ deep without cell or flare. No iris rubeosis was visible in either eye.
IOP measured 44mm Hg O.D. and 18mm Hg O.S. Gonioscopy revealed appositional closure 360 degrees in the right eye, but portions of the angle did open with compression gonioscopy. The left eye was open to scleral spur.
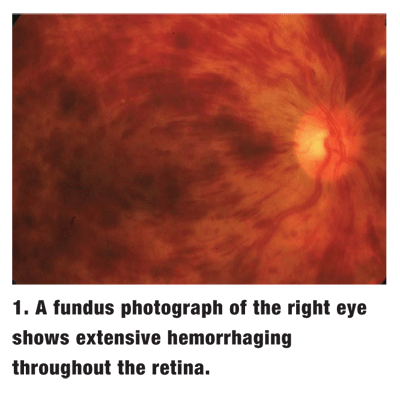
Dilated fundus exam showed extensive hemorrhages throughout the retina in the right eye (figure 1). The left eye was completely normal.
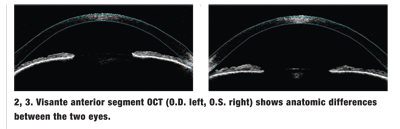
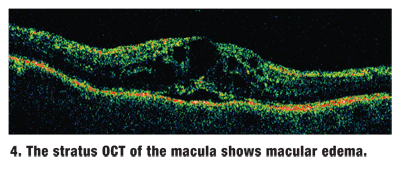
We also ordered Visante anterior segment optical coherence tomography (OCT) of both eyes (figures 2 and 3), and Stratus OCT of the macula (figure 4).
Take the Retina Quiz
1. What does the fundus image of the right eye represent?
a. Central retinal vein occlusion.
b. Proliferative diabetic retinopathy.
c. Ocular ischemia.
d. Lymphoma.
2. What does the Visante OCT of the anterior segment show?
a. Iris bomb configuration.
b. Angle closure.
c. Forward rotation of the lens/iris diaphragm.
d. All of the above.
3. What is the cause of the elevated IOP?
a. Angle closure secondary to anatomically narrow angle.
b. Angle closure secondary to rubeotic glaucoma.
c. Angle closure secondary to ciliary body congestion.
d. Open-angle glaucoma secondary to overproduction of aqueous.
4. What is the appropriate initial treatment for this patient?
a. Pilocarpine 4% q.i.d.
b. Atropine 1% b.i.d.
c. Laser peripheral iridotomy.
d. Laser iridoplasty.
For answers, see below.
Discussion
Our patient has what appears to be a fairly classic central retinal vein occlusion (CRVO) in the right eye. There is generalized congestion of the optic nerve, with dilated and tortuous retinal veins and hemorrhages in all four quadrants. Clinical examination and OCT of the macula revealed macular edema.
Of particular interest is the IOP of 44mm Hg in the right eye. The most likely cause in a CRVO is neovascular glaucoma. Surprisingly, neovascular glaucoma was not the cause in our patient.
Approximately 70% of CRVOs are classified as perfused, while the remaining 30% are ischemic in nature. Patients with ischemic CRVOs have the greatest riskabout 35%of developing neovascularization of the iris (INV) and angle (ANV).1 These patients require close and careful follow-up, because once they develop INV and ANV, they can go on to develop neovascular glaucoma, which is one of the most devastating forms of glaucomaand perhaps the most difficult to treat.
When examining these patients, pay close attention to the iris frill. Also, perform gonioscopy, looking closely for vessels in the angle. Panretinal photocoagulation (PRP) is indicated for patients who develop INV or ANV.
We ordered fluorescein angiography for our patient; however, we were unable to determine if the CRVO was ischemic or perfused due to the amount of hemorrhage. When this occurs, the CRVO is classified as indeterminate. As the hemorrhage resolves, the nature of the CRVO becomes more apparent.
Careful exam of our patients iris did not reveal any neovascularization on the iris frill. Because the patient has appositional closure (i.e., no angle structure was visible on standard noncompression gonioscopy), it was difficult to determine if there was any neovascularization in the angle. On compression gonioscopy, portions of the angle were able to be opened, and no ANV was seen. So, where does that leave us?
An important clue: Our patient has a very narrow AC, both peripherally and centrally. Additionally, when you compare the depth of the AC in that eye to the left eye, you see that the left eye is very deep, and the iris is flat. The iris in the affected eye bows forward and has an iris bomb configuration. This occurs when the aqueous cannot exit the angle because of closure. The pressure gradient from the posterior chamber to the AC increases, and the iris bows forward.
We ordered a Visante anterior segment OCT, which clearly shows the anatomic differences between the right and left eyes. The iris of the left eye is flat, and the angle is open. Also, the anterior surface of the lens, which is barely seen, is in its normal position, posterior to the iris. In the right eye, however, we see a dramatic bowing of the iris toward the cornea and complete closure of the angle. The anterior lens surface is much more forward in the right eye than the left eye.
So, the mystery is how this all fits together. In rare instances of CRVO, congestion and swelling of the ciliary body can occur, probably due to an abnormal accumulation of blood and fluid. When this happens, there is a forward rotation of the ciliary body that results in anterior displacement of the lens iris diaphragm; this, in turn, causes a secondary angle closure.2,3 The forward rotation has also been reported to occur following retinal de-tatchment repair and following PRP. That scenario is what has happened in our patient.2,3
In typical angle closure glaucoma, the treatment is to instill a miotic, such as pilocarpine. However, pilocarpine would only make this condition worse as it continues to try and pull the iris diaphram (and ciliary body) forward. The best treatment is to dilate the pupil with a cycloplegic, which relaxes the ciliary body and allows aqueous to flow freely from the posterior to the anterior segment.
A laser peripheral iridotomy (LPI) is also done in traditional angle closure, but not in this form of angle closure. For these patients, an argon laser peripheral iridoplasty (ALPI) can be performed at the junction where the angle is closed. Contraction of the laser burns typically results in mechanical opening of the angle.
Our patient was treated with atropine b.i.d. and ALPI. We also managed him with topical glaucoma medications. Within a week, the angle returned to its normal depth, and IOP was maintained at 17mm Hg. Subsequently, the patient had two intravitreal Avastin injections, which resulted in resolution of his macular edema and a final visual acuity of 20/100 O.D. We had hoped for a greater improvement in acuity, but unfortunately, that does not always happen after a CRVO, despite resolution of the edema. This is often due to ischemia and/or permanent structural damage in the photoreceptors.
Retina Quiz Answers: 1) a; 2) d; 3) c; 4) b.
1. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol 1997 Apr;115(4):486-91.
2. Shields MB. Glaucoma Associated with Disorders of the Retina, Vitreous, and Choroid. In: Shields MB. Textbook of Glaucoma. 4th ed.
3. Mendelsohn AD, Jampol LM, Shoch D. Secondary angle closure after central retinal vein occlusion. Am J Ophthalmol 1985 Oct 15;100(4):581-5.