A 79-year-old white female has been followed since 2000, when she first presented as a glaucoma suspect. At her initial visit, she was diagnosed with ocular hypertension, but she demonstrated normal optic nerve appearances and visual field studies.
A 79-year-old white female has been followed since 2000, when she first presented as a glaucoma suspect. At her initial visit, she was diagnosed with ocular hypertension, but she demonstrated normal optic nerve appearances and visual field studies.
She was monitored until 2005, when her right eye converted to glaucoma. The conversion manifested as a change in her neuroretinal rim. The left eye ultimately converted to glaucoma in January 2007, due to a similar neuroretinal rim change.
We stabilized the patients glaucomatous optic neuropathy with one drop of Lumigan (bimatoprost, Allergan) O.U. h.s. and one drop of 0.1% Alphagan P (brimonidine tartrate, Allergan) O.D. b.i.d.
Her current systemic medications include pravachol, Accupril (quinapril, Pfizer), vitamins and a calcium supplement.
Diagnostic Data
Her best-corrected visual acuity in early October 2008 measured 20/40- O.D. and 20/40 O.S. Her anterior segments have always been unremarkable, except for corneal arcus O.U.
The patients pupils were equally round and reactive to light and accommodation, with no afferent pupillary defect. Her extraocular motilities were full in all positions of gaze.
Through dilated pupils, her crystalline lenses were characterized by grade 3 nuclear cataracts and grade 2 anterior and posterior cortical cataracts O.D., and grade 2 nuclear and cortical cataracts O.S. The cataracts have progressed gradually during the past several years, which accounts for her decreased best-corrected acuity. The reduced visual acuity, she reported, did not interfere with her quality of life.
Since being medicated with Lumigan and Alphagan P, her IOP has consistently ranged from 12mm Hg to 16mm Hg O.D. and 13mm Hg to 15mm Hg O.S. Her pretreatment IOP averaged 24mm Hg O.D. and O.S.
Gonioscopy had consistently demonstrated grade 3 open angles at each visit during the last few years, with moderate trabecular pigmentation. Pachymetry readings were 531m O.D. and 530m O.S. Her optic nerves were characterized by cup-to-disc ratios of 0.55 x 0.65 O.D. and 0.35 x 0.45 O.S., and have remained stable since we initiated therapy.
Threshold visual fields demonstrated an early paracentral arcuate defect superiorly O.D. and normal fields O.S.
Unfortunately, during the last two follow-up visits, her IOP increased to 25mm Hg O.D. and 22mm Hg O.S. So, what should we do now?
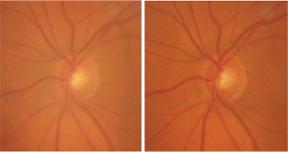
A stereo view of our 79-year-old female patients left eye demonstrates glaucomatous damage.
Discussion
There are several possible reasons for our patients decreased IOP control, including poor or inadequate compliance. Or, her glaucoma might simply be becoming more difficult to manage. But, in this case, I thought that her cataracts were at least partially responsible for the increased IOP. As her cataracts have progressed over the past few years, her anterior chamber has become progressively more shallow due to the increased thickening of the crystalline lenses.
Although there was no evidence of pupillary block, posterior synechiae or iris bomb, the anterior chamber angles had clearly narrowed. At the visit in early October 2008, gonioscopy demonstrated grade 1 to 2 open angles O.U., as opposed to grade 3 a year ago.
Regarding cataract surgery, our office protocol is to explain the options to the patient and ascertain if the cataracts are affecting his or her vision. If the patient is visually debilitated, we proceed with cataract surgery; if he or she is not, then we simply defer cataract surgery.
In this case, however, even though the patient did not have significant visual complaints, I explained that having her cataracts removed would facilitate better IOP control by de-bulking the anterior chamber. She agreed to proceed with surgery.
It is extremely important for glaucoma patients to control IOP immediately following cataract surgery to decrease the potential for postoperative complications. It is well known that topical steroids have the potential to increase IOP, especially in glaucoma patients.1
Because it is crucial to maintain tight control of IOP during the postoperative period, glaucoma patients need to continue using their medications both prior to and immediately following cataract surgery. With that said, we must also address the possibility of postoperative complications of cataract surgery associated with glaucoma medicationsin particular, prostaglandin analogues.
As we know, prostaglandins have the potential to increase anterior chamber inflammation in some patients and possibly increase the risk of cystoid macular edema (CME). Additionally, surgical trauma to the eye can induce anterior uveitis and CME.
When prostaglandins first became available, we used to discontinue the prostaglandin prior to cataract surgery, substitute another glaucoma medication, assess IOP stability, and then perform cataract surgery once stability was assured. During that time, we did not document any increase in the severity of postoperative anterior uveitis and/or CME.
Recently, though, there has been a shift to continue prostaglandin use directly up to surgery, and then resume using them immediately afterward.2 We have successfully made that paradigm shift in our office protocol without any noticeable increase in the severity and/or risk of postoperative CME.
Ultimately, our patient underwent cataract surgery in her right eye on October 7, 2008. Unfortunately, she was confused about her postoperative drop regimen and stopped using both Lumigan and Alphagan the day after surgery. I was curious as to why this previously compliant patient, who had been medicating herself with glaucoma drops for several years, would stop using her drops. She told me that the Lumigan and Alphagan were not on the postoperative schedule of medications given to her by the cataract surgeon. In hindsight, I can see how that could happen, and will make protocol modifications to prevent it from occurring in the future.
Not surprisingly, at her one-week post-op visit, her uncorrected acuity was 20/25- O.D., but her IOP measured 32mm Hg. Given a relatively quiet anterior chamber at this one-week visit, I elected to taper the dosage of Pred Forte (prednisolone acetate, Allergan) a bit more quickly than normal, as well as restart both the Lumigan and Alphagan.
We scheduled her for follow-up in two weeks, at which time her IOP should be lower. At this early point, however, it remains to be seen whether the cataract surgery has accomplished its main goal: to facilitate better IOP control.
1. Levkovitch-Verbin H, Habot-Wilner Z, Burla N, et al. Intraocular pressure elevation within the first 24 hours after cataract surgery in patients with glaucoma or exfoliation syndrome. Ophthalmology 2008 Jan;115(1):104-8.
2. Chang JH, McCluskey P, Missotten T, Ferrante P, et al. Use of ocular hypotensive prostaglandin analogues in patients with uveitis: does their use increase anterior uveitis and cystoid macular oedema? Br J Ophthalmol 2008 Jul;92(7):916-21.