
A 64-year-old white female, Lucy, presented to our office as a referral from a local optometrist. The referring optometrist was concerned about a spike in intraocular pressure (IOP) secondary to a possible angle closure of the right eye.
Lucy had initially sought care because of pain and decreased vision in her right eye. She described the pain as a deep ache that had increased from a slight discomfort over the previous few days to a more bothersome sensation on the day of her visit.
She had also noted vision loss the day before the exam by chancebecause of the pain in her right eye, she compared vision between it and her left eye and noticed the discrepancy.
History
Lucys medical history was positive for Type II diabetes mellitus, hypercholesterolemia and hypertension. She had been diagnosed with diabetes 12 years ago and reported her most recent blood sugar level as 180 milligrams per deciliter. She was unsure of the last glycosylated hemoglobin value.
Her current medications included metformin and nateglinide for diabetes, lovastatin for elevated cholesterol and lisinopril for hypertension. She was unsure of her last blood pressure or cholesterol measurements, though she reported both as controlled.
Diagnostic Data
Upon examination, her habitual visual acuity was 20/60 O.D. and 20/30 O.S. Pupillary responses were graded as sluggish O.D. and normal O.S., with no afferent pupillary defect. Extraocular muscles functioned without restrictions.
Intraocular pressure measured 28mm Hg O.D., and between 16mm Hg and 17mm Hg O.S. Biomicroscopy was unremarkable in the right eye, but the left eye revealed a mild limbal flush, clear cornea, quiet anterior chamber and grade 4+ neovascularization of the iris. Large-caliber vessels were present and bridged the pupil in several locations.
Gonioscopy revealed an angle open to the scleral spur 360 O.U., with vessels throughout the angle of the right eye. Undilated examination of the posterior segment O.S. showed scattered hemorrhages in all four quadrants. The right eye displayed retinopathy equivalent to that in the left eye, as well as signs of a possible old superior hemiretinal vein occlusion with sclerosed vessels and collateral vessels. There was also evident macular edema.
Diagnosis
We diagnosed Lucy as being at high risk for proliferative diabetic retinopathy. We also diagnosed her with ocular hypertension O.D. secondary to mechanical obstruction of the angle by neovascularization of the iris (NVI), rather than true neovascular glaucoma.
Treatment and Follow-Up
We referred the patient to a retinal specialist that day for an intravitreal injection of Avastin (bevaci- zumab, Genentech) and possible panretinal photocoagulation.
We instructed Lucy to return to our clinic in four days for a follow-up, and we began her on a regimen of Alphagan P (brimonidine tartrate, Allergan), one drop t.i.d. O.D., and timolol 0.5%, one drop b.i.d. O.D., to use until the follow-up appointment.
The report from the retinal surgeon confirmed our assessmentbut, while an intravitreal injection of Avastin had been given, panretinal photocoagulation was deemed unnecessary.
At the first follow-up, Lucy said that her eye felt more normal, although her vision was still reduced O.D. She reported compliance with her eye drop regimen and denied any side effects other than mild irritation upon instillation.
Her visual acuity was measured at 20/50 O.D. and 20/30 O.S. Pupils were normal and demonstrated no afferent pupillary defect. Her IOP was 12mm Hg O.D. and 16mm Hg O.S. Biomicroscopy and gonioscopy revealed that the neovascularization of the iris was receding and that abnormal vasculature was absent from the angle. We assessed the NVI as resolving, discontinued her eye drops and scheduled a second follow-up in one week.
When Lucy returned for her second follow-up, her visual acuity was stable from the prior week; IOP was 17mm Hg O.U.; and the NVI had completely resolved.
We planned to bring Lucy back for a full work-up in two weeks in order to determine appropriate treatment, but at this point, the patient was lost to follow-up.
Discussion
Thanks to the emerging ophthalmic use of anti-vascular endothelial growth factor (anti-VEGF) drugs, conditions that were once impossible to treat without significantly damaging the retina, such as NVI, are now being managed effectively and without fear of permanent retinal damage. This class of drugs appears to be rewriting the management strategies for virtually all vascular proliferative disorders of the eye.
Vascular endothelial growth factor is the name given to a family of proteins that play a major role in moderating angiogenesis (the development of new blood vessels) in both the normal and pathologic state. VEGF was first identified from tumor ascites of a guinea pig in 1983, at which point it was simply known as vascular permeability factor.1 During this investigation, it was found that expression of vascular permeability factor resulted in a temporary increase in vascular permeability 50,000 times of that induced by histamine. When further studies deemed VEGF the only stimulant for vascular endothelial invasion of tissue, the proteins influence as an angiogenic factor was fully realized.2
Increased expression of VEGF has been implicated as the major cause of neovascularization in the human eye with exudative age-related macular degeneration.3 Despite its role in major ocular pathology, VEGF is present in the normal eye at lower levels. The concentration has been found to be particularly high in the retinal pigment epithelium, where its expression likely helps to support the natural fenestrations of the choriocapillaris.3
In the hypoxic eye, VEGF increases in concentration not only within the neural retina, but also in the vitreous and aqueous.4 This increased level of VEGF correlates strongly with levels of hypoxia, and when ischemia is reversed (e.g., by panretinal photocoagulation), VEGF levels drop and rapidly approach normal levels.5 The increased expression of VEGF not only promotes the development of pathologic blood vessels, but also appears to be fundamental in both the breakdown of the blood retinal barrier and the development of macular edema.
The blood retinal barrier is dependent on the tight junctions of retinal vasculature and a lack of the fenestrations characteristic of choroidal circulation.6 VEGF disrupts this system by breaking down tight junctions, stimulating leukocyte-induced damage of vessel walls and forming fenestrae not unlike those of the choriocapillaris.3 Upon decreasing the concentration of VEGF, these changes can be reversed.4
VEGF Control
Studies that demonstrate suppression of tumor growth with use of anti-VEGF drugs helped inspire the development of bevacizumab as a treatment for colorectal cancer. Even prior to Avastins release in the market, animal studies of Lucentis (ranibizumab, Genentech), a smaller, genetically altered fragment of the same molecule as bevacizumab, showed promise: significant inhibition of choroidal neovascularization in the ocular tissue of rhesus monkeys.7 The initial choice of ranibizumab and Macugen (pegaptanib sodium injection, Eyetech/Pfizer), for development over bevacizumab was based on an earlier study in which a molecule the same size as bevacizumab (although of different molecular make-up) was unable to sufficiently penetrate into the retina to exert a substantial therapeutic effect.8 So, early trials of intravitreal anti-VEGF drugs utilized genetically altered fragments of the antibody rather than the full-length bevacizumab molecule.
As early as 2004, the VEGF Inhibition Study in Ocular Neovascularization (VISION) study group demonstrated positive effects of Macugen when compared to sham therapy in the phase III clinical trial.9 But, patients who received the injection continued to lose vision over the course of the study, though at a slower rate than the control group.
Shortly thereafter, investigations utilizing ranibizumab looked even more promising. Ranibizumab is a fragment of bevacizumab, and at roughly one-third the size, it may be expected to demonstrate superior retinal penetrance and nearly four times the binding affinity of bevacizumab. The
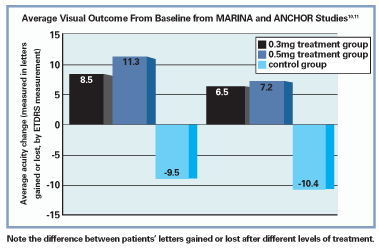
For example, in the
Results of the ANCHOR study, which examined the efficacy of photodynamic therapy vs. Lucentis injections in managing AMD with predominantly classic choroidal neovascular nets, were also positive. Both treatment groups receiving ranibizumab (0.3mg and 0.5mg) experienced, on average, a net gain in vision and an average reduction in size of the choroidal neovascular membrane (CNVM) by roughly 0.55 disc areas, while a net vision loss and 0.54 disc area increase in lesion size occurred with PDT. In addition, the results were so favorable that during the phase III arm of the study, ranibizumab was made available to the PDT-only group.11
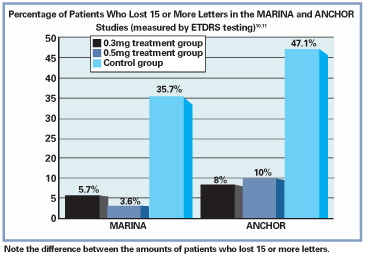
The safety profile of ranibizumab was also shown to be reliable, though its use has been linked with conjunctival hemorrhages, pain, transient increased IOP, mild cataract development and, more seriously, increased risk of endophthamitis and inflammation.10,11 Systemically, cerebral vascular accident and myocardial infarction risks were reported within the studies, although the incidence in the ranibizumab group was not found to be significantly higher than that in the control group.10,11
Meanwhile, Avastin became available for the treatment of colorectal cancer in February of 2004. In 2005, Philip Rosenfeld, M.D., Ph.D., the pioneer of intravitreal bevacizumab use, realized that by using this readily available supply, he could prepare the molar equivalent of a ranibizumab dose at a wholesale cost between $17 and $50. While the costs to a patient using bevacizumab for monthly chemotherapy treatment is $4,400, the wholesale cost for a 4ml vial of the medication is only $550. This translates into an approximate cost to the patient of $150 per injection, vs. between $1500 and $2,000 per injection, which a patient receiving Lucentis can expect to pay.12
Other data presented by William L. Rich III, M.D., at the American Academy of Ophthalmology 2006 meeting showed the following costs for one years use of different AMD treatments: $1,019 for Avastin, $5,581 for photodynamic therapy, $13,638 for Macugen and $33,564 for Lucentis.13 In an analysis by William Smiddy, M.D., the cost per line of visual acuity saved per year utilizing various treatments for occult choroidal neovascular lesions of AMD were found to be $551 for PDT, $1,248 for Macugen, $900 for Lucentis, and only $60 for Avastin.13
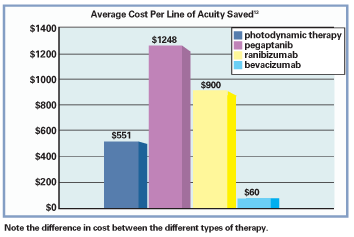
With the enormous difference in cost, it was only a matter of time before bevacizumab itself was investigated as a treatment for choroidal neovascularization and wet AMD.14 Results demonstrated a substantial improvement of vision and a dramatic decrease of central retinal thickness from the original edematous state of the pathologic macula. The safety profile was found to be excellentthe lone reported side effect was a rare transient increase in systolic blood pressure that dropped to normal levels within 12 weeks with medical management.14
The first published case of intravitreal Avastin use describes findings that are strongly supportive.15 The patient presented with longstanding subfoveal neovascularization that was unresponsive to three separate treatment regimensPDT, verteporfin with intravitreal triamcinolone, and Macugen. She elected to undergo an experimental injection of Avastin. At one week, her macular OCT, fundoscopic appearance and fluorescein angiography were all improved. By four weeks, OCT and angiographies had returned to normal appearance.15 The study group pointed out that these findings contradict the previously published assumption that bevacizumab would exhibit insufficient retinal penetrance due to its relatively large molecular size.8
Also, many anecdotal experiences have demonstrated results as promising in managing wet AMD as those of ranibizumab, at a fraction of the cost. Currently, the National Eye Institute has funded a trial on the clinical comparison of the effectiveness of bevacizumab (fixed and variable dosing) vs. ranibizumab (fixed and variable dosing) in managing patients with wet AMD: the Complications of Age-Related Macular Degeneration Treatment Trials.11
Other Uses of Avastin
Most clinical research on ophthalmic applications of anti-VEGF drugs has focused on wet AMD, but Avastins potential to treat any ocular vasculoproliferative disorder has not been lost on retinal specialists. In a poster presented at the American Academy of Ophthalmology, results from roughly 1,300 intravitreal injections of bevacizumab at the New York Eye and Ear Infirmary were reported.13
Following these, patients with AMD, macular edema, neovascular glaucoma or rubeosis irides all showed positive results, with no cases of endophthalmitis reported.13
Anti-VEGF Limitations
VEGF inhibitors are only indicated in cases of active wet AMD where vision loss is not due to scarring. Further, a subgroup analysis of the
A second negative predictor of visual outcome was found to be larger-sized baseline neovascular lesions.14 The effect of the net size may be due to a relative immunity to VEGF suppression, which older neovascular nets develop.
Studies analyzing structural changes in chorioretinal neovascular beds have described a leukocytic remodeling of pathologic vessels that takes place weeks after its initial growth.16 This remodeling appears to make the vasculature resistant to the effects of VEGF suppression. In such cases, it would be expected that, while VEGF suppression may decrease permeability of the vessels and inhibit further angiogenesis, the original lesion will not exhibit the extensive regression that it would have prior to the remodeling.16 Therefore, a lesion of greater size (and greater age) may be anticipated to regress less than a smaller lesion and would be a negative predictor of visual outcome.
What Can You Do?
Between 2001 and 2004, intravitreal injections in the United States increased from 4,500 to around 83,000, a large component of which was due to intravitreal anti-VEGF injections.13 The PrONTO study group attempted to outline the appropriate dosing regimen of ranibizumab and found that, after one injection every month for three months, further dosing could be guided empirically based on OCT and fluorescein angiography.17 Due to the limited number of retinal specialists and the increasing number of intravitreal injections, optometrists are likely going to see more of these patients for follow-up, and only when ocular examination or dosing schedules warrant will they be referred back to the specialist for further injection.
The anti-VEGF class of medications is revolutionizing the treatment of vasculo-proliferative disorders, and their appeal is instantly recognizable. Over the past year, the press has published several articles regarding the potential of anti-VEGF medications, and our patients will latch onto the reviews of these medications. For example, the day after a local newspaper had published a brief article discussing Lucentis, my clinic received 14 phone calls regarding the medication from people living with various sight-threatening eye diseases who wanted to know if they would be candidates for treatment.
The point: It is our responsibility as eye-care professionals to educate ourselves regarding powerful new treatment options so that we can, in turn, further educate our patients and subsequently make informed referrals and management decisions.
Dr. Bronner practices at
1. Senger DR, Galli S, Dvorak A, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983 Feb 25;219(4587):983-5.
2. Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989 Dec 8;246(4935):1306-9.
3. Adamis AP, Shima DT. The role of VEGF in ocular health and disease. Retina 2005 Feb-Mar;25(2):111-8.
4. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994 Oct 15;118(4):445-50.
5. Shinoda K, Ishida S, Kawashima S, et al. Clinical factors related to the aqueous levels of vascular endothelial growth factor and hepatocyte growth factor in proliferative diabetic retinopathy. Curr Eye Res 2000 Aug;21(2):655-61.
6. Remminton L. Clinical Anatomy of the Visual System.
7. Adamis AP, Shima DT, Tolentino MJ, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol 1996 Jan;114(1):66-71.
8. Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol 1999 Sep-Oct;27(5):536-44.
9. Gragoudas ES, Adamis AP, Cunningham ET, et al. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration.
10. Rosenfeld PJ, Brown DM, Heier JS, et al. The MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration.
11. Brown DM,
12. Steinbrook R. The price of sightranibizumab, bevacizumab and the treatment of macular degeneration.
13. Brown D. Conference Report. In: Highlights of the
14. Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 2005 Jun;112(6):1035-47.
15. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after bevacizumab for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2005 Jul-Aug;36(4):331-5.
16. Boyer DS, Antoszyk AN, Awh CC, et al. The MARINA Study Group. Subgroup analysis of the
17. Fung A, Lalwani G, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007 Apr;143(4):566-83.











