ABSTRACT: Bladder cancer is one of the most common cancers. When a diagnosis is made, the majority of cases have superficial disease and are low grade in nature. The management of bladder cancer is multifaceted and includes not only surgical intervention but also pharmacologic management with intravesical treatment. Bladder cancer has a high likelihood of recurrence; therefore, knowledge of tumor staging, treatment guidelines, and vigilant follow-up is critical in prevention of recurrence and progression.
In 2008, bladder cancer was diagnosed in approximately 68,000 individuals in the United States.1,2 The disease is four times more prevalent in men than in women and is more common in Caucasians than in African-Americans or Hispanics. There are significant costs associated with bladder cancer due to its prevalence and the intense monitoring required to detect tumor recurrence. The management of bladder cancer includes surgical intervention and intravesical therapy.
There are several risk factors that have been identified for bladder cancer.3,4 Cigarette smoking is the most common and is linked to over half of bladder cancer cases. Smokers are reported to have a two- to fourfold increase in risk compared to nonsmokers. Although this risk decreases with smoking cessation, it never truly returns to the level of a nonsmoker.4 Additional risk factors include environmental or occupational exposure to urothelial carcinogens, such as aromatic amines used in rubber, leather, and dye industries.5 This type of exposure accounts for 5% to 20% of bladder cancer cases. Medical risk factors include prior pelvic irradiation, chemotherapy with cyclophosphamide, and chronic urinary tract infections. A positive family history for bladder cancer is also considered a risk factor.
Symptoms and Diagnosis
The most common symptom associated with bladder cancer is hematuria (80%), with 20% to 30% of patients also reporting dysuria, increased urgency, and increased frequency.5
Patients presenting with such symptoms are typically evaluated with cystoscopy for lesion detection and potential biopsy. If lesions are seen on cystoscopy, patients are scheduled for transurethral resection of the bladder tumor (TURBT) and for staging.6 Staging of bladder cancer is reflective of the depth at which the tumor has penetrated the bladder wall. Stage I cancer is limited to the inner lining of the bladder, while Stage IV disease extends to adjacent organs, the abdominal wall, or the wall of the pelvis (TABLE 1).

In the majority (93%) of presenting cases, bladder cancer is a transitional cell carcinoma (TCC). Less than 5% are squamous cell carcinomas, and 2% are adenocarcinomas. The majority (75%) of cases are superficial or nonmuscle-invasive disease.5
Urologists typically consider patients in one of two groups: those who have muscle-invasive bladder cancer and those who do not.7 Unfortunately, approximately 4% of patients present with metastatic disease with some involvement of the lymph nodes, lung, liver, bone, or central nervous system.8
Treatment
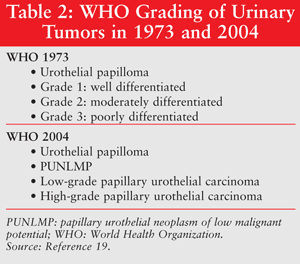
Treatment options for bladder cancer are dependent upon staging, the results of a TURBT, and previous treatments. It is important to understand the various tumor-grading types to appreciate the treatment options. Tumor grading (TABLE 2) is necessary to assess recurrence and potential for progression. The World Health Organization (WHO) 1973 grading system assigns tumors into well-differentiated, moderately differentiated, or poorly differentiated cancers. In 2004, this system was revised to differentiate between papillary urothelial neoplasms of low malignant potential (PUNLMP) and low-grade and high-grade urothelial carcinomas. However, there should be more uniform diagnosis of tumors that is better stratified according to risk potentials, and this grading system needs to be validated in more clinical trials before it is used widely. Under the primary tumor, regional lymph node, and distant metastasis (TNM) grading system (TABLE 1), nonmuscle-invasive bladder cancer (NMIBC) includes Ta, Tis, and T1 tumors. At the time of diagnosis, NMIBC accounts for 75% to 80% of bladder tumors and the remaining are muscle invasive. Within NMIBC, about 70% present as Ta tumors, 20% as T1 tumors, and 10% as Tis tumors.9
Unfortunately, when it comes to treatment of NMIBC there is no single consensus guideline. Instead, the four main organizations have released their recommendations for the treatment and management of bladder cancer. These include the European Association of Urology (EAU), the First International Consultation on Bladder Tumors (FICBT), the National Comprehensive Cancer Network (NCCN), and the American Urological Association (AUA). These reviewing bodies share similar philosophies, yet they maintain their own set of recommendations. In all cases, the guidelines center around two main modes of treatment: TURBT and intravesical therapy.
Transurethral Resection of Bladder Tumor
All treatment guidelines consider TURBT the gold standard for NMIBC. Complete TURBT is useful not only to diagnose bladder lesions, but also serves as the cornerstone in management and follow-up. The initial TURBT is used to define histologic type, grade, and depth of invasion. This helps determine prognosis and allows for design of adjuvant therapy and follow up. As mentioned, Ta tumors are the most common, with a 10-year disease-specific survival after TURBT of 85%. Ten-year survival for T1 tumors is 70%.7 A repeat TURBT procedure at 4 to 6 weeks after the original resection may be necessary for patients with multiple tumors or in high-risk patients with T1 or higher grade tumors to ensure complete resection and to verify that recurrence is not due to residual tumor. In some cases, restaging can occurred with repeat TURBT.
Intravesical Therapy
The goal of bladder cancer therapy is to reduce the recurrence rate and prevent tumor progression. After TURBT, intravesical therapy is often used as adjuvant or maintenance therapy. This can be done by using chemotherapeutic or immunomodulatory agents.
Intravesical Chemotherapeutic Therapy
Guidelines support the use of an immediate single-instillation dose of intravesical chemotherapy following TURBT. This is usually done within 24 hours of TURBT and preferably within 6 hours. It is thought that chemotherapy can aid to destroy floating tumor cells and prevent reimplantation in the bladder. It can reduce the risk of recurrence to about 50% in the short term and to about 15% in 5 years.10 It is important to note that the benefits of a single chemotherapy instillation have not been studied in patients with high-risk bladder cancer. Intravesical chemotherapy should be avoided in cases of overt or suspected bladder wall perforation, due to the potential for systemic absorption of the chemotherapeutic agent. Agents commonly used for NMIBC are doxorubicin, epirubicin mitomycin C, and triethylenethiophosphoramide (thiotepa). Although similar in efficacy, they have different side-effect profiles.Doxorubicin has been used in the management of bladder cancer; however, the side-effect profile limits its use. Dose is not well defined, but 50-mg instillation has been use in clinical trials. Chemical cystitis can occur in up to 56% of patients and hematuria in up to 40%, followed by a decreased bladder capacity in 16% and other systemic reactions in 5%.11 There have also been reports of the use of epirubicin, a synthetic derivative of doxorubicin. It is therapeutically similar to that of doxorubicin but it has a better side-effect profile. Reports of cystitis and hematuria are less significant than with doxorubicin.11 It should be noted that epirubicin is not FDA approved for intravesical use in the U.S.
Mitomycin C is also commonly used at a dose of 40 mg in 60 mL distilled water. It is an alkylating agent and its molecular weight is higher at 334 kDa, thus it has less systemic absorption. The most common side effect noted with mitomycin C is dysuria and urinary frequency. Newer chemotherapy agents such as gemcitabine and docetaxel have also been used; however, long-term efficacy data is still lacking.
Thiotepa is an alkylating agent known to cross-link DNA and prevent cells from replicating. Due to its high side-effect profile, thiotepa (30 mg in 30-mL distilled water) is not commonly utilized in the U.S. It is small in molecular weight (189 kDa) and readily absorbed into systemic circulation. Myelosuppression can be up to 54%, and incidence of irritative voiding symptoms can be up to 69%.11
Intravesical Immunotherapy
Mycobacterium bovis was initially isolated in 1904 from a cow with tuberculosis mastitis. In 1908, Calmette and Guerin worked with it in an attempt to develop an antituberculosis vaccine (named M bovis bacillus Calmette-Guerin, BCG). It was not until the end of the 19th century, when it was observed that patients with tuberculosis rarely developed malignant neoplasms, that BCG was explored as an anticancer agent.8 However, BCG given systemically as IV was not proven to be an effective cancer treatment option. To be effective, cancer has to be localized as much as possible (small tumor burden), and BCG has to have direct contact with the tumor cells.12 These very conditions are present in superficial bladder cancer. In 1976, Morales et al first used BCG, administered intravesically, for successful treatment of NMIBC following TURBT.13 There are various BCG strains. The most widely used substrain is Connaught, Pasteur, Tice, and Tokyo.14
A live attenuated strain of M bovis is instilled into the bladder. Once instilled, the mycobacteria attach to the bladder's urothelial lining. This causes an inflammatory reaction, which leads to the activation of macrophages and CD4 T-lymphocytes. Cytokine-mediated immune response is then induced, which results in a release of interferon, tumor necrosis factor, and interleukin--all of which appear to be toxic to cancer cells.14
Dosage of BCG: BCG is usually started 4 to 6 weeks postoperatively. An optimal dose and schedule, which has not been clearly defined, ranges from 50 mg to 81 mg per instillation. Although strains of BCG have been studied at varying doses, with good results, large-scale studies are needed to define an optimal dose. Reconstitution of BCG is done with 50 mL of sterile, preservative-free saline in a syringe. It is then instilled intravesically with a urethral catheter. The medication is held in the bladder for 2 hours (TABLE 3). The induction dosing schedule is usually every week for 6 weeks. A maintenance schedule has not been defined. The current BCG maintenance schedule is based on the Southwest Oncology Group (SWOG) regimen of three-weekly instillations at 3 and 6 months, followed by every 6 months for 3 years.15 Tolerance of BCG therapy has not been shown to affect compliance with the full course. In a randomized trial with BCG maintenance therapy using the recommended schedule, only 16% of the patients completed the planned cycles due to significant number of side effects.16

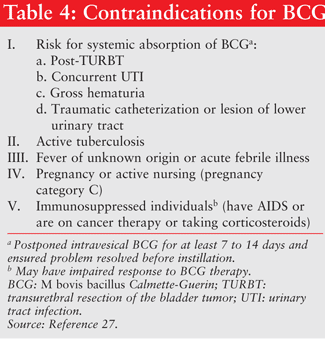
Side Effects of BCG: BCG should not be given if contraindications are identified (TABLE 4). Side effects associated with BCG include both local and systemic reactions. Local adverse events include lower urinary tract symptoms (57%-71%), hematuria (20%), and bladder contracture (3%).14 Systemic events include fevers, chills, and flu symptoms (30%), and epididymitis, prostatitis, and urethral infection (4%). Other reported events are skin rash and low-grade fever. High fever can occur in association with multiple organ failure in generalized BCGitis.17 Management of side effects to BCG therapy can include dosage reduction, increasing the instillation interval, or reducing dwell time from 2 hours to less than 30 min. However, there is no long-term efficacy data to support using these methods.18 Pharmacologic therapy with NSAIDs, urinary analgesics, and antispasmodics can be used for most BCG-associated cystitis symptoms. Hospitalization and triple tuberculosis therapy (isoniazid, rifampin, ethambutol) and high-dose corticosteroid are required for BCGitis.17
Management Based on Risk
Management of Low-Risk Bladder Cancer
Characteristics of low-risk bladder cancer include a tumor that is small in volume and low-grade stage-Ta cancers. It is also defined as G1-2Ta cancer with low risk of recurrence and progression.5 Typically, cancer recurrence rates are 15% in 1 year and 31% in 5 years, with a progression rate of 0.2% at 1 year and 0.8% at 5 years in this group.19 All guidelines recommend TURBT with a single postoperative intravesical chemotherapy instillation within 24 h. Mitomycin C or doxorubicin is usually used. Their use has been shown to reduce the risk of recurrence in NMIBC by 50% at 2 years and greater than 15% at 5 years.20 Meta-analysis of randomized trials showed that a one-time immediate instillation of chemotherapy after TURBT can result in 12% reduction in tumor recurrence.15
Management of Intermediate-Risk Bladder Cancer
Intermediate-risk is defined as multifocal or large-volume, low-grade Ta or recurrent low-grade Ta. It can also be characterized as G2Ta, G1T1, or G2T1 with intermediate or high risk of recurrence and intermediate risk of progression.5 The risk of recurrence is higher than the risk of progression, with a progression risk of 5% at 1 year and 17% at 5 years.19 Guidelines recommend TURBT and a single immediate postoperative instillation of chemotherapy. Treatment recommendations recognize that adjuvant intravesical treatment with either mitomycin C or BCG is necessary in intermediate-risk disease, but there is no consensus within the guidelines regarding frequency or duration of therapy.
The EAU recommends induction of BCG plus maintenance for at least 1 year or intravesical chemotherapy for 6 to 12 months.20 At this time, the AUA only recommends an induction course of intravesical BCG or mitomycin C. They do, however, recognize that maintenance therapy can reduce recurrences more effectively than induction alone. Meta-analysis showed that TURBT plus BCG maintenance can reduce recurrence by 31% and TURBT plus mitomycin C reduces recurrence by 18%.21 Both cost and risk of side effects may outweigh the benefit of treatment, which is why the AUA considers maintenance therapy with either regimen as optional treatment.21 After TURBT and postoperative instillation of chemotherapy, the FICBT recommends less than 6 months of intravesical chemotherapy as first-line therapy and BCG as second-line therapy.22 In contrast, the NCCN did not make any specific recommendations other than TURBT. After TURBT, observation can be initiated or intravesical therapy with BCG (preferred) or mitomycin C can be considered.23
Management of High-Risk Bladder Cancer
High-risk cancer is define as high-grade Ta or T1, or highly recurrent carcinoma in situ. It can also be described as multiple G2T1, G3Ta-T1 with high risk of progression.5 The recurrence rate in this category is 61% in 1 year and 78% in 5 years. The progression rate is 17% at 1 year and 45% at 5 years.19
The goal of management in high-risk bladder cancer is to prevent or delay any progression to muscle-invasive disease. All available guidelines recommend the initial TURBT and a second TURBT in 2 to 6 weeks, especially if a complete resection was not done or was not possible the first time. Adjunctive intravesical therapy is important in this category. Regarding the choice of agent for intravesical therapy, BCG is considered the standard of treatment in high-risk disease. BCG is superior to mitomycin C for reducing tumor progression. Meta-analysis by the AUA confirms the superiority of BCG to mitomycin C with or without maintenance. The estimated 5-year recurrence rate was 34% in patients receiving TURBT followed by BCG maintenance compared to 62% with mitomycin C maintenance.10 Another meta-analysis evaluated randomized controlled trials comparing BCG after TURBT versus TURBT alone in medium- to high-risk patients with Ta and T1 bladder cancer. This showed that treatment with BCG after TURBT significantly reduced the number of patients with recurrence at 12 months after TURBT and also delayed the time to recurrence.24 As a result, BCG is the treatment of choice for preventing and or delaying bladder cancer progression to muscle-invasive disease.
The EAU recommends at least a year of intravesical BCG after a second TURBT. Radical cystectomy should be considered in highest risk patients that have recurrent high-grade tumor, high-grade T1, or high-grade tumors with carcinoma in situ (CIS). For CIS disease, the EAU recommends at least 1 year of BCG therapy. Patient assessment to response should be done in 3 months. If no response is found after 6 months, a cystectomy is recommended.20 The FICBT also recommends a second TURBT follow by a 6 weeks of BCG induction with 1 to 3 years of BCG maintenance therapy. For CIS disease, BCG maintenance is recommended by the FICBT. Although the schedule and duration is not specified, it should be noted that it should be more than1 year in the absence of treatment failure.22,25 According to the NCCN, repeat TURBT is recommended if a complete resection is uncertain the first time. BCG is generally preferred after repeat resection.25 Lastly, the standard treatment according to the AUA is to repeat the resection followed by both induction BCG plus maintenance therapy.21
Treatment Failures
There are no standard definitions for treatment failures. The EAU defines failures as muscle invasion or high-grade nonmuscle invasion at follow-up or any worsening of disease after BCG treatment. The AUA does not offer a definition of failures but does provide recommendations for management if cancer has reoccurred after intravesical therapy. Regardless of whether there is one definition for all to follow, bladder cancer recurrence and progression is still a problem. The EAU recommends a trial of intravesical BCG instillation for NMIBC recurrence after intravesical chemotherapy. For high-grade, nonmuscle-invasive tumors that re-occurred at 3 months following BCG therapy, another course of BCG can be given. Otherwise, cystectomy is highly recommended for high-risk BCG failures due to risk of progression to muscle and risk of metastasis.20 The FICBT recommends cystectomy in patients with recurrence of high-grade disease after BCG induction therapy, in failure before maintenance BCG has been completed, in early failure after the completion of maintenance BCG therapy, and in recurrent T1 tumors if patients have had two prior induction cycles of BCG. For those who refuse cystectomy, another course of BCG can be given.22 The AUA, on the other hand, recommends repeat resection for high-grade cancer that has recurred after intravesical therapy. Once resected, another course of intravesical therapy (BCG) is recommended. They too recommend early cystectomy as a therapeutic alternative before progression to muscle-invasive disease.21 Intravesical valrubicin was recently FDA approved for treatment of BCG refractory CIS in patients who refuse cystectomy. Other alternatives are intravesical gemcitabine or intravesical BCG with alpha 2b interferon. The efficacy of these treatment options are still under investigation.
Radical Cystectomy
Radical cystectomy is indicated for high-grade disease recurrence after intravesical BCG and CIS refractory to immunotherapy. By definition, high-grade T1 and CIS have the potential to progress and metastasize. Cystectomy should be done before disease progression involves muscle invasion. Timing of radical cystectomy is critical. Patients with early cystectomy are reported to do better clinically than those with late cystectomy, who may have pathologic evidence of muscle and extravesical involvement and metastatic disease.26
Follow-Up
It is not surprising that there are different recommendations for follow up as well. The EAU recommends surveillance cystoscopy at 3 months for low-risk patients. If recurrence is negative at 3 months, then a repeat cystoscopy should be done at 9 months and annually for 5 years. In high-risk patients, the recommendation is initial cystoscopy at 3 months repeated every 3 months for 2 years, every 4 months in the third year, then every 6 months until the 5th year and annually thereafter. Annual upper-tract imaging is also important in this group. Scheduled follow up for intermediate-risk patients should be between that of low-risk and high-risk disease.20 The FICBT schedule for follow up is similar to that of the EAU.22 The AUA, on the other hand, recommends cystoscopy every 3 months for the first 2 years, every 6 months in third year, then annually thereafter. Beside cystoscopy, an assessment of voiding symptoms, hematuria history, urinalysis, and urine cytology are also needed during follow-up periods.10,21 The NCCN recommends upper-tract imaging every 1 to 2 years along with cystoscopy and urine cytology every 3 months for 2 years, followed by imaging every 6 months for the next 2 years. Screening is recommended annually thereafter.23
Summary
Bladder cancer is one of the most common cancers today, with over 500,000 Americans living with the diagnosis. The majority of cases are superficial bladder cancer, yet there are still patients who present with lesions that have invaded the muscle wall. Treatment options are dependent upon patient risk category, staging, and previous treatments. Management of bladder cancer is multifaceted and includes pharmacologic management with intravesical treatment. Following initial treatment, vigilant follow-up is critical since the prognosis is poor for those with metastatic disease. Unfortunately, bladder cancer does have a high likelihood of recurrence, further emphasizing the importance for ongoing evaluation and follow-up.
REFERENCES
1. US National Institutes of Health, National Cancer Institute. Bladder Cancer. www.cancer.gov/cancertopics/
2. Konety BR, Joyce GF, Wise M. Bladder and upper tract urothelial cancer. J Urol. 2007;177:1636-1645.
3. Creel. Bladder cancer: epidemiology, diagnosis, and treatment. Sem Onc Nurs. 2007;23(4)(Suppl 3):S3-S10.
4. Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000;86:289-294.
5. Colombel M, Soloway M, Akaza H, et al. Epidemiology, staging, grading and risk stratification of bladder cancer. Eur Urol Suppl. 2008;7:618-626.
6. Halling KC, Kipp BR. Bladder cancer detection using FISH (UroVysion assay). Adv Anat Pathol. 2008;15:279-286.
7. Dalbagni G. The management of superficial bladder cancer. Nat Clin Practice Urol.8. Brandau S, Suttmann H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother. 2007;61:299-305.
9. Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4-34.
10. Hall MC, Chang SS, Dalbagni G, et al. Guidelines for the management of non-muscle invasive bladder cancer (stages Ta, Ta and TIS): 2007 update. J Urol. 2007;178:2314-2330.
11. Thrasher JB, Crawford ED. Complication of intravesical chemotherapy. Urol Clin North Am.
12. Heney NM, Koontz WW, Barton B, et al. Intravesical thiotepa versus mitomycin C in patients with Ta, T1 and TIS transitional cell carcinoma of the bladder: a phase III prospective randomized study. J Urol. 1988;140:1390-1393.
13. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180-183.
14. Razack AH. Bacilus Calmette-Guerin and bladder cancer. Asian J Surg. 2007;30(4):302-309.
15. Persad R, Lamm D, Brausi M, et al. Current approaches in the management of non-muscle invasive bladder cancer: comparison of current guidelines and recommendation. Eur Urol Suppl. 2008;7:637-650.
16. Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124-1129.
17. Rischmann P, Desgrandchamps F, Malavaud B, Chopin DK. BCG intravesical instillation: recommendations for side-effects management. Eur Urol. 2000;37(suppl 1):33-36.
18. Braasch MR, Bohle A, O'Donnell MA. Risk-adapted use of intravesical immunotherapy. BJU Int. 2008;102:1254-1264.
19. Sylvester RJ, van de Meijden APM, Oossterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466-477.
20. Babjuk M, Oosterlinck W, Sylvester R, et al. European Association of Urology guidelines on TaT1 (non-muscle invasive) bladder cancer. Arnhem, The Netherlands: European Association of Urology; March 2008:1-21.www.uroweb.org. Assessed February 23, 2009.
21. American Urological Association. Guidelines for the management of non-muscle invasive bladder cancer (stages Ta, T1 and Tis): 2007 undate. Linthicum, MD: AUA; 2007. www.auanet.org. Assessed February 23, 2009.
22. Nieder AM, Brausi M, Lamm D, et al. Management of stage T1 tumours of the bladder: international consensus panel. Urology. 2005;66:108-125.
23. National Comprehensive Cancer Network. Clinical practice guideline in oncology: bladder cancer including upper tract tumours and urothelia carcinoma of the prostate. Version 1. Jenkintown, PA: NCCN; 2007.
24. Shelley MD, Hynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guerin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001;88:209-216.
25. Sylvester RJ, van der Meijden AP, Witjes JA, et al. High grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology. 2005;66:90-107.
26. Bianco FJ Jr. Management of clinical T1 bladder transitional cell carcinoma by radical cystectomy. Urol Oncol. 2004;22:290-294.
27. Boyd LA. Intravesical bacillus Calmette-Guerin for treatment bladder cancer. Urol Nurs. 2003;23(3):189-199.
To comment on this article, contact
[email protected].











