US Pharm.
2006;31(7)(Oncology suppl):3-15.
Skin
cancer is the most commonly diagnosed malignancy in the United States. Over
one million cases of squamous cell and basal cell skin cancers are projected
to be diagnosed in 2006.1 The most deadly form of skin cancer is
malignant melanoma. The American Cancer Society (ACS) estimated that 62,190
new cases of cutaneous melanomas would be diagnosed in the U.S. in 2006, with
7,910 deaths from the disease. Melanomas are responsible for about 4% of all
new cancers and about 1% of cancer deaths per year.1
The lifetime risk of
developing a cutaneous melanoma is one in 63 for American men and women.2
The age-adjusted incidence is 17.2 per 100,000 people annually, with a higher
incidence in men (21.8 per 100,000) than in women (14 per 100,000). The median
age at diagnosis is 57 years. The annual percentage increase of melanomas was
6.1% from 1975 to 1981 and 2.8% from 1981 to 2002. Melanomas are most common
among white Americans (25.9 per 100,000 males, 17.2 per 100,000 females),
followed by Hispanics (4.5 per 100,000 males, 4.4 per 100,000 females). They
are least common among African-Americans (1.3 per 100,000 males, 0.8 per
100,000 females).
Risk Factors
Melanomas are usually found on sun-exposed areas. In men, the most common location for a melanoma is on the trunk, head, or neck, whereas women are most likely to develop lesions on their legs. Sun exposure is a well-known risk factor for melanomas. The nature of sun exposure influences the risk. A review of case-control studies on sun exposure and melanoma risk revealed a positive association with intermittent exposure, a history of sunburn at all ages, and a smaller link with total sun exposure.3 A reduced risk of melanoma is associated with occupational exposure, as reported in a recent meta-analysis examining sun exposure and melanoma risk.4 In addition, there is an association between melanomas and the use of tanning beds and sun lamps.5
Although melanomas are known to
develop in a preexisting lesion, such as a mole, they can develop on any area
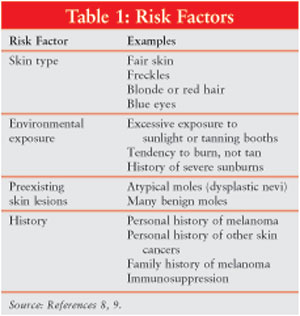
of the skin. Risk factors for melanomas include skin type, environmental
exposure, preexisting skin lesions, personal and family history, and
immunosuppression (Table 1).
Individuals with atypical moles (dysplastic nevi) have a higher risk of
developing the cancer. Atypical moles are often large, flat, and asymmetrical
and have ill-defined borders.6 Atypical moles can be located on any
area of the skin but are most common on the upper back. These moles may be
brown, tan, black, pink, or red. The risk of melanoma is directly correlated
with the number of dysplastic nevi.
Prevention
There are two types of melanoma
prevention: primary and secondary. The goal of primary prevention is to
prevent the development of the disease. To reduce the risk of melanoma, it is
important to be protected from the sun's damaging influence. Protection can be
attained through the implementation of basic principles of safe sun practices:
1. During the peak
midday period between 10 am and 4 pm, avoid exposure to strong sunlight. If
outdoors, stay in the shade during these times. Avoid deliberate tanning with
sun lamps and tanning beds.
2. Prevent sun damage
with sunscreens that protect against ultraviolet light. Sunscreens should have
an SPF rating of at least 15 and should be applied liberally and frequently
during outdoor activities.
3. When outdoors, wear
sunglasses and protective clothing (e.g., long-sleeved shirts and wide-brimmed
hats).7
The goal of secondary
prevention is to discover the disease in its earliest, most curable form. One
of the best methods to detect melanomas is an examination of the skin.
Professional assessments and self-examinations are important components of
early melanoma detection. Self-examinations should be performed regularly in a
private, well-lit area. A full-length mirror and a hand-held mirror are useful
when examining areas difficult to see, and a blow dryer may be helpful when
evaluating the scalp. Several organizations, such as the ACS and American
Academy of Dermatology (AAD), have free brochures that describe and illustrate
how to perform a self-examination for skin cancers. In addition, the ACS and
AAD both demonstrate how to conduct a skin self-examination at their Web sites
(www.cancer.org and www.aad.org, respectively).
Pathology
Most melanomas begin as superficial
lesions within the epidermis and may remain there for years.8 They
grow primarily in a horizontal manner, which is known as the radial growth
phase. During the radial phase, melanomas grow slowly and are classified
as a melanoma in situ, a lesion confined to the epidermis, or as a microinvasive
melanoma, which grows horizontally in the epidermis but may also spread
microscopically into the dermis. During the vertical growth phase, the
melanoma begins to spread deeply into the dermis. This phase is more
dangerous, since the disease can spread to other areas of the body, such as
lymph nodes and other tissues. Most melanomas are characterized by a radial
growth phase that may eventually evolve into a vertical growth phase. The
major exception is nodular melanoma, which has no radial growth phase and
starts in the vertical growth phase.
The four major melanoma
subtypes, in order of frequency, are superficial spreading, nodular, lentigo
maligna, and acral lentiginous melanoma.8 Superficial spreading
melanomas account for 60% to 70% of all melanomas.9 Although
this type can appear anywhere, it is commonly found on the trunk in men and on
the legs in women. These melanomas appear as pigmented lesions that may
contain a number of colors, including black and different shades of brown.
Usually, superficial spreading melanomas have an irregular border with a
notched or scalloped appearance.
Nodular melanomas represent
15% to 30% of all melanomas and are the most aggressive, exhibiting no radial
growth.9 They may appear as a dark nodule on the skin. Nodular
melanomas are common on the trunk or head and neck and are more common in men.
Representing 5% of all
melanomas, lentigo maligna melanomas are often found on the face of
elderly individuals.9 They may appear as a flat, brown or tan
lesion that may be quite large.
The least common melanoma is acral
lentiginous, which accounts for less than 5% of melanomas.9
Most often, this type is found on the palms of the hands and soles of the feet
but can also be seen under fingernails or toenails. Acral lentiginous
melanomas may be brown or black or contain color variations.8,9
Presentation
Melanomas
characteristically present as a pigmented lesion on the skin. Melanomas may
develop in a preexisting lesion; however, the majority develop in previously
normal areas of the skin. The ACS developed the "A-B-C-D" system to identify
typical characteristics of a melanoma.10 "A," or asymmetrical,
refers to the shape of the lesion. Most benign lesions are symmetrical,
whereas melanomas are often asymmetrical. "B" refers to the lesion's border,
which is usually irregular in melanomas. "C" refers to color, specifically
color variation. The color of the melanoma may range from a solid, dark lesion
to a lesion with several different shades, including blue, black, brown, pink,
white, gray, or red. "D" refers to the diameter of the lesion. Most melanomas
are more than 6 mm in diameter, which is about the size of a pencil eraser.
Recently, "E" has been added to the scheme, representing evolution. Any change
in a preexisting lesion, including a change in size, color, swelling, border,
or raised area, should be evaluated.
Diagnosis
An accurate diagnosis of a
suspicious lesion can be made only through a biopsy. Small lesions can be
removed through an excisional biopsy, which removes the lesion and a
small margin of surrounding normal skin. If the lesion is too large to remove
or is located in a delicate area, it may be examined through an incisional
biopsy that removes only part of the lesion. The biopsy should be a
full-thickness biopsy that includes subcutaneous tissue. The pathologist can
then determine the thickness and depth of the lesion.
Varying levels have been
established to identify the relationship between penetration depth and risk
for metastasis. The deeper the penetration, the more likely it is to spread.
Clark's system is used only to assess prognosis in patients with thin lesions
and does not correlate with prognosis and tumor thickness. It comprises five
levels:
Level I: Limited
to the epidermis.
Level II: Extending
to the papillary dermis but not filling it.
Level III:
Filling the papillary dermis.
Level IV: Infiltrating
into the reticular dermis.
Level V:
Involving subcutaneous fat.
Breslow classified melanomas according to
their thickness and reported a good correlation between thickness and
prognosis. An additional negative prognostic factor is the presence of
ulceration.8
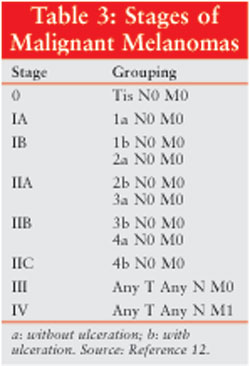
Staging
After a diagnosis
is made, the patient should be evaluated to determine the stage or extent of
disease. Once the extent is known, the appropriate treatment can be initiated,
and an estimation of the patient's prognosis can be made. Staging includes an
evaluation of the entire skin surface, regional lymph nodes, chest/abdominal
CT scans, and serum lactate dehydrogenase (LDH). Stages are defined according
to the American Joint Committee on Cancer's TNM (tumor, node, metastasis)
system (Tables 2 and 3).11 "T" represents the primary
tumor and is divided into substages based on thickness (T1 to T4) and the
absence or presence of ulceration ("a" and "b," respectively). "N" represents
lymph node involvement and contains three levels based on the number of nodes
involved with disease. "M" indicates the presence or absence of distant
metastases, with subsets based on the location of the lesion(s) and the serum
LDH level.

Melanomas may be classified as
localized disease (83% at diagnosis), regional disease (11%), and advanced
disease (3%), with the stage of the remaining 3% unknown.2
Localized disease, including stages I and II, has not spread beyond the area
where it developed. Regional disease (stage III) occurs when the tumor has
spread to local lymph nodes. Advanced disease (stage IV) is characterized by
spread to distant parts of the body.
Surgery
Surgical removal of a melanoma is
performed through a wide excision that removes the lesion and an area of
surrounding normal tissue. The margin of tissue removed is determined by the
thickness of the tumor. A margin of 1 cm is recommended for Ia lesions.
Lesions that are 1.1 to 2 mm thick should be resected with margins of 1 to 2
cm; lesions that are greater than 2 mm in thickness require margins of 2 cm.12
Patients with stage Ia
melanomas are usually managed with wide excision and careful follow-up. Wide
excision is also appropriate for patients with stage Ib and stage II tumors.
However, lymph node evaluation is often recommended for these patients.
Evaluation of the sentinel lymph node is a means of evaluating one lymph node
and, if negative, may spare the patient a more extensive lymph node
dissection. The sentinel lymph node is considered the first node that the
disease will spread to once malignant cells leave the tumor and enter the
lymphatic system. The node is identified by injecting a blue dye and a
radiopharmaceutical agent into the tissue surrounding the primary tumor. The
sentinel lymph node will be the first node to take up the dye. If the node is
negative, the patient can be assigned to observation or adjuvant therapy. If
positive, dissection of regional nodes should be performed.
Patients with clinically
positive nodes (stage III) undergo wide excision of the primary tumor with
complete lymph node dissection, followed by close observation or adjuvant
therapy. Patients who present with distant metastases (stage IV) can be
treated with systemic therapy.
Adjuvant Therapy
After surgery, adjuvant therapy is
administered. Systemic therapy is aimed to treat any remaining microscopic
disease to prevent relapse. Adjuvant therapy for early-stage melanoma is
controversial.
Interferon
Alfa:Although a
number of treatments have been used, there is no standard that has been
definitively proven to extend overall survival. A commonly recommended
adjuvant therapy is interferon alfa. The initial interest in adjuvant
interferon alfa was sparked by the Eastern Cooperative Oncology Group (ECOG)
E1684 trial (discussed in detail below).13 Based largely on the
results of this trial, interferon alfa-2b was approved for adjuvant therapy in
adults with melanoma who are disease-free but at high risk for recurrence.
Four phase III randomized
trials evaluated the activity of high-dose interferon alfa.14-17
The first study compared three months of high-dose interferon alfa-2a to
observation.8 There was no difference in disease-free or overall
survival between treatment and observation. This was followed by three studies
of interferon alfa-2b conducted by the ECOG. In the first study (E1684),
patients were randomized to high-dose interferon at doses of 20 million units
(MU)/m2 for five days per week intravenously for one month,
followed by 10 MU/m2 three days per week subcutaneously for 48
weeks, or observation.13 After a median follow-up of 6.9 years, a
significant difference in respective overall survival (3.8 vs. 2.8 years) was
seen between the interferon and observation groups. However, at a median
follow-up of 12.6 years, the difference in overall survival was insignificant.15
Toxicity was significant and included neutropenia, flu-like symptoms, fatigue,
depression, and hepatotoxicity. Dose reduction or delays were required in 37%
of the patients during the induction phase and were required in 36% during the
maintenance phase of the trial.
The E1684 trial was followed
by a three-arm trial (E1690) that compared the same interferon dose and
schedule to low-dose interferon and observation.16 High-dose
interferon was associated with an increase in relapse-free survival, compared
to observation, but there was no overall survival benefit in either interferon
arm. A lack of survival benefit may have been due to the fact that patients in
the observation arm were allowed to receive interferon therapy upon relapse.
The third study (E1694)
randomly compared high-dose interferon to a melanoma vaccine called GMK,17
which contains ganglioside GM2, a melanoma antigen that is the most
immunogenic ganglioside expressed on melanoma cells. The study was halted
early when a midpoint analysis revealed a significantly higher disease-free
and overall survival in the interferon arm. A pooled analysis of updated data
from all three ECOG trials revealed no significant impact of high-dose
adjuvant interferon on overall survival, compared to observation.15
Due to the toxicity
encountered with high-dose interferon, a number of randomized trials compared
low-dose interferon to observation. Interferon was primarily administered at a
dose of 3 MU three times per week for six months to three years.18
Disease-free survival was significantly improved with interferon only in two
studies,19,20 and none of the studies demonstrated an improvement
in overall survival.
Since high-dose interferon was
associated with significant toxicity and low-dose interferon did not improve
survival, a phase III study of intermediate-dose interferon was initiated.21
Patients received 13 or 25 months of interferon alfa-2b versus observation.
The 13-month regimen had no significant impact on survival, and the 25-month
regimen improved survival by only 5.4%.
These studies demonstrate that
there is no compelling evidence to suggest interferon alfa as the adjuvant
therapy of choice in patients with stage IIb/III disease at intermediate to
high risk of recurrence. Guidelines proposed by the National Comprehensive
Cancer Network suggest offering participation in a clinical trial, high-dose
interferon alfa-2b, or observation to patients with localized lesions more
than 4 mm thick who are at significant risk for recurrence or who have
positive lymph nodes.10
Isolated Limb Perfusion
In-transit
metastases are lesions that develop in cutaneous and subcutaneous lymphatic
vessels between the primary tumor and lymph nodes. Their incidence is 5% to 8%
in patients with high-risk melanomas.22 They are surgically excised
if their number and location allow removal without excess morbidity. Relapse
after surgical excision is common. Dong et al. reported that 55% of 648
patients experienced a second regional relapse after surgical excision within
two years, and 82% relapsed by five years.23
In-transit metastases located
on extremities may be treated with isolated limb perfusion (ILP). In this
procedure, which is performed under general anesthesia, the affected limb is
surgically isolated from the rest of the body with a bypass system.
Chemotherapeutic agents are infused into the limb, concentrating the drugs in
the limb and sparing the rest of the body from the effects of the drugs. The
limb is also heated (hyperthermia) to improve the activity of the drugs. The
most commonly employed drug for ILP is melphalan. The overall response rate to
melphalan with hyperthermia (>=40oC) ranges from 80% to 90%,
with complete response rates as high as 55% to 65%.22 Responses
generally last about nine to 12 months. Toxicities include local skin
erythema, peripheral neuropathy, and myopathy.
Distant Metastatic Disease
Patients with
advanced-stage melanomas may be treated with chemotherapy, immunotherapy, or
both. Responses to systemic therapy are classified as complete (no evidence of
disease), partial (more than a 50% reduction in size of primary tumor), minor
(less than a 50% reduction in size of primary tumor), or stable (no change).
Overall response combines complete and partial responses.
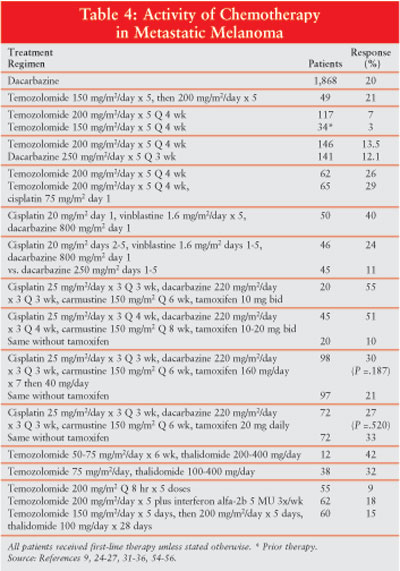
Dacarbazine and
Temozolomide:The most
active chemotherapeutic agent is dacarbazine (DTIC). DTIC is associated with
an overall response rate of about 20% (Table 4), and the duration of
response to single-agent DTIC is about four to six months.9 DTIC is
administered intravenously or by infusion. Major adverse effects associated
with DTIC are anorexia, nausea and vomiting, myelosuppression, local tissue
damage upon extravasation, and infrequent occurrence of a flu-like syndrome.
Temozolomide, a prodrug that
degrades to the active agent MTIC, is also the active metabolite of DTIC. It
has the advantages of oral administration and better penetration into the
central nervous system. It has been administered as a single agent to over 400
patients with advanced melanoma and resulted in response rates of 6% to 26%,
mostly in patients without prior therapy.24-27 Median overall
survival ranged from 5.5 to 11.5 months.
Temozolomide and DTIC were
compared in a head-to-head trial of 305 patients and produced respective
response rates of 13.5% and 12.1% and survivals of 7.7 and 6.4 months,
respectively.28 Temozolomide was also compared with combination
temozolomide and cisplatin, and no significant differences were found in
respective response rates (26% vs. 29%) or survival (11.5 vs. 12 months).27
However, in another study, temozolomide plus cisplatin produced a response
rate of 48.6% among 37 patients.28 Temozolomide was administered
with interferon in two trials involving a total of 87 patients, with response
rates of 12.5% and 27.6%. Survivals were only 11.8 and 14.5 months,
respectively.29,30
Adverse effects with
temozolomide include myelosuppression, mild to moderate nausea and vomiting,
headache, and fatigue. Unfortunately, despite the advent of newer agents such
as temozolomide, no single agent has demonstrated significantly better
outcomes than DTIC. However, temozolomide, since it is administered orally, is
more convenient and better tolerated than DTIC regarding nausea and vomiting
and does not carry the risk of extravasation.
Combination Chemotherapy:
DTIC has been
combined with many agents for the management of advanced melanoma. One of the
most active regimens is cisplatin, vinblastine, and DTIC (CVD). The first
trial of this regimen produced an objective response rate of 40% over a
nine-month median duration of response among 50 patients with advanced disease.31
This combination was then compared to DTIC alone in patients with advanced
disease. The most recent data from this study revealed no significant
differences in respective response rates (19% vs. 14%), median duration of
response (21.5 vs. 17 weeks), or median survival (27 vs. 21 weeks) between CVD
and DTIC alone.32
A four-drug regimen, known as
the Dartmouth regimen, contains cisplatin, DTIC, carmustine, and tamoxifen.
The first study of this therapy indicated a response rate of 46%, including an
11% complete response rate.33 In another small trial involving 20
patients, a response rate of 50% was reported. However, concern about the
possibility of DVT and PE prompted researchers to delete tamoxifen from the
regimen, and the response declined to 11%. When tamoxifen was reinserted into
the regimen, the response was 52%.34 A combined analysis of these
trials is presented in Table 4.34 A response rate of 51% was
found in 45 patients who received CVD plus tamoxifen, whereas 20 patients who
received CVD without tamoxifen achieved a 10% response.34 Despite
the differences in response with and without tamoxifen, there was no survival
difference between the regimens.
Two randomized studies
compared the original four-drug regimen to the three-drug regimen without
tamoxifen and reported no significant difference in response or survival,
thereby eliminating the need for tamoxifen in this combination.35,36
A phase III study compared the Dartmouth regimen to single-agent DTIC and
reported slightly higher response rates with the combination (18.5%) over DTIC
alone (10.2%). However, the difference was not significant and there was no
survival benefit with the combination regimen.37
Interferon Alfa:
Although the exact mechanism of action is unknown, interferon alfa is thought
to exert its antineoplastic effects partly via modulation of the immune
system. Over a dozen trials of single-agent interferon involved about 400
patients with advance melanomas. Doses ranged from 10 MU/m2/day to
50 MU/m2 three times per week. Response rates ranged from 4% to
29%, with an average overall response of 16% (4% complete).9 The
median duration of response was only four months. Interferon was no more
active than DTIC and represented considerable toxicity and cost.
The combination of interferon
and DTIC has produced response rates of 17% to 46%.38-41 Several
trials compared DTIC alone to DTIC plus interferon. Falkson randomized 73
patients to DTIC alone or DTIC plus interferon.38 Seven patients
responded to DTIC (19%) and 17 to the combination (46%)--a significant
difference. The combination was also associated with a significantly longer
time to treatment failure (nine vs. 2.5 months) and median survival (16.7 vs.
eight months). However, the activity of DTIC plus interferon was not confirmed
in three other comparative trials.39-41 A major adverse effect is a
flu-like syndrome that may begin in hours to days after administration. Signs
and symptoms may include fever, chills, arthralgias, myalgias, fatigue,
headache, and malaise. This reaction may be dose-related and is seen in most
people who receive this drug.
Interleukin-2: Interleukin-2
(aldesleukin) is one of the few agents approved for management of metastatic
melanoma. As with interferon, the exact antineoplastic mechanism of
interleukin-2 is unknown but is thought to involve immunomodulation. A review
of single-agent trials of 270 patients who received high-dose interleukin-2
(720,000 U/kg/dose) reported an overall response rate of 16%.42 The
median duration of response for all who responded was 8.9 months, and the
median overall survival for the entire population was 11.4 months. Twenty of
the responding patients (47%) survived after a median follow-up of 62 months,
including 15 who survived more than five years. Interleukin-2 is associated
with significant toxicities that may limit its use. Combination interleukin-2
and interferon alfa has produced response rates of 11% to 24%.43-45
A phase III study randomized 85 patients with advanced disease to
interleukin-2 alone or in combination with interferon alfa-2a.46
There was a 2% response among patients who received interleukin-2 alone,
versus 10% among those who received interleukin-2 plus interferon--an
insignificant difference. These studies report no survival benefit to
combination interferon alfa and interleukin-2.
Interleukin-2 has been linked
to serious and sometimes life-threatening side effects, which may limit its
use to select young patients with a good performance status. Most patients
with advanced melanoma do not fit this description and would likely not be
candidates. The frequency and severity of these effects are dose-related. A
black box warning in the prescribing literature states that an intensive care
facility and specialists in cardiopulmonary or intensive care medicine must be
available.47
Biochemotherapy:
Biochemotherapy combines chemotherapy and immunotherapy. Many of these
regimens contained a chemotherapy base of DTIC to which other chemotherapy
agents are added. The combination of DTIC plus interferon has produced
response rates of 17% to 28% and median survivals of 4.5 to 17 months.48
The addition of two to three more chemotherapeutic agents has increased
response rates but has not had an impact on survival. DTIC and interleukin-2
has produced responses in 16% to 26% of patients, with survivals of nine to 13
months.48 Combination interferon alfa and interleukin-2 has been
administered with a variety of chemotherapeutic agents. Response rates have
been as high as 64%, but survivals remain in the eight- to 12-month range.48
Only two phase III trials of biochemotherapy reported significant differences
in response with biochemotherapy, one over immunotherapy and the other over
chemotherapy.49,50 However, no significant differences in survival
were found in any of the studies. The combination of chemotherapy plus
immunotherapy may increase the response over either treatment alone but at a
cost of greater toxicity with no clearly significant survival benefit.
Thalidomide: Thalidomide
has demonstrated immunomodulatory and antiangiogenesis activity with benefit
in patients with multiple myeloma and other malignancies.51
Although trials of single-agent thalidomide against metastatic melanomas
reported no clinical responses,52,53 it has been combined with
temozolomide and interferon alfa with mixed results.54-56 Adverse
effects include constipation, peripheral neuropathy, dizziness, somnolence,
edema, dry skin, dry mouth, tremor, and fatigue.
Summary
Malignant melanoma is the most
dangerous form of skin cancer. The incidence of melanoma continues to
increase. Exposure to ultraviolet light is a major risk factor that can be
minimized through safe sun practices. Melanomas are readily detectable on the
skin surface. People should be encouraged to perform self-examinations and to
report any abnormality to their physician.
Surgical excision of early lesions offers
the best opportunity for a cure. Interferon alfa is the only agent approved
for adjuvant therapy in patients at risk for relapse after surgery, but
recommendations for adjuvant therapy are controversial. Treatment of advanced
disease remains disappointing. The most active single agent is DTIC, which is
associated with about a 20% response rate. Although many combination regimens
have been investigated, none offers superior survival benefits over DTIC. The
addition of interferon alfa and/or interleukin-2 to chemotherapy may increase
response rates but with no impact on survival. Five-year survivals are 98.3%
with localized disease, 63.8% with regional disease, and 16% in patients with
metastatic disease.
References
1. Jemal A, et al. Cancer
statistics, 2006. CA Cancer J Clin. 2006;56:106-130.
2. SEER data. Accessed at
seer.cancer.gov/statfacts/html/melanhtml on December 3, 2005.
3. Elwood JM, Jopson J. Melanoma and
sun exposure: an overview of published studies. Int J Cancer.
1997;73:198-203.
4. Gandini S, et al. Meta-analysis of
risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer.
2005;41:45-60.
5. Gallagher RP, et al. Tanning beds,
sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol
Biomark Prev. 2005;14:562-566.
6. Naeyaert JM, Brochez L. Dysplastic
nevi. N Engl J Med. 2003;349:2233-2240.
7. Ramirez R, Schneider J. Practical
guide to sun protection. Surg Clin N Am. 2003;83:97-107.
8. Liu V, Mihm MC. Pathology of
malignant melanoma. Surg Clin N Am. 2003;83:31-60.
9. Lotze MT, et al. Cutaneous
melanoma. In Devita VT Jr., Hellman S, Rosenberg SA, eds. Cancer Principles
and Practice of Oncology. 6th ed. Philadelphia: Lippincott Williams &
Wilkins; 2001:2012-2069.
10. Friedman RJ, et al. Malignant
melanoma in the 1990s: The continued importance of early detection and the
role of physician examination and self-examination of the skin. CA Cancer J
Clin. 1991;41:201-226.
11. Balch CM, et al. An evidence-based staging
system for cutaneous melanoma. CA Cancer J Clin 2004;54:131-149.
12. NCCN National Comprehensive
Cancer Network Clinical Practice Guidelines in Oncology, Melanoma version.
Available at: www.NCCN.org. Accessed February 2006.
13. Kirkwood JM, et al. Interferon
alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern
Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7-17.
14.Creagan ET, et al. Randomized,
surgical adjuvant trial of recombinant interferon alfa-2a in selected patients
with malignant melanoma. J Clin Oncol. 1995;13:2776-2783.
15. Kirkwood JM, et al. A pooled
analysis of Eastern Cooperative Oncology Group intergroup trials of adjuvant
high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670-1677.
16. Kirkwood JM, et al. High- and
low-dose interferon alfa-2a in high-risk melanoma: first analysis of
intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444-2458.
17. Kirkwood JM, et al. High-dose
interferon alfa-2b significantly prolongs relapse-free and overall survival
compared with the GM-2KLH/QS-21 vaccine in patients with resected stage
IIB-III melanoma: Results of intergroup trial E1694/S9512/C509801. J Clin
Oncol. 2001;19:2370-2380.
18. Brown CK, Kirkwood JM. Medical
management of melanoma. Surg Clin N Am. 2003;83:283-322.
19. Grob JJ, et al. Randomised trial
of interferon alfa2a as adjuvant therapy in resected primary melanoma thicker
than 1.5 mm without clinically detectable node metastases. Lancet.
1998;351:1905-1910.
20. Pehamberger H, et al. Adjuvant
interferon alfa-2a treatment in resected primary stage II cutaneous melanoma.
Austrian Malignant Melanoma Cooperative Group. J Clin Oncol.
1998;16:1425-1429.
21. Eggermont AM, et al. Post-surgery
adjuvant therapy with intermediate doses of interferon alfa 2b versus
observation in patients with stage IIb/III melanoma (EORTC 18952): randomised
controlled trial. Lancet 2005;366:1189-1196.
22. Eggermont AMM, et al. The role of
isolated limb perfusion for melanoma confined to the extremities. Surg Clin
N Am 2003;83:371-384.
23. Dong XD, et al. Analysis of
prognosis and disease progression after local recurrence of melanoma. Cancer.
2000;88:1063-1071.
24. Bleehen NM, et al. Cancer
Research Campaign phase II trial of temozolomide in metastatic melanoma. J
Clin Oncol. 1995;13:910-913.
25. Agarwala SS, et al. Temozolomide
for the treatment of brain metastases associated with metastatic melanoma: a
phase II study. J Clin Oncol. 2004;22:2101-2107.
26. Middleton MR, et al. Randomized
phase III study of temozolomide versus dacarbazine in the treatment of
patients with advanced metastatic malignant melanoma. J Clin Oncol.
2000;18:158-166.
27. Bafaloukos D, et al. Temozolomide
and cisplatin versus temozolomide in patients with advanced melanoma: a
randomized phase II study of the Hellenic Cooperative Oncology Group. Ann
Oncol 2005;16:950-957.
28. Daponte A, et al. Temozolomide
and cisplatin in advanced malignant melanoma. Anticancer Res.
2005;25:1441-1447.
29. Richtig E, et al. Temozolomide
and interferon alpha 2b in metastatic melanoma stage IV. Br J Dermatol.
2004;151:91-98.
30. Ridolfi R, et al. Temozolomide
and interferon-alpha metastatic melanoma: a phase II study of the Italian
Melanoma Intergroup. Melanoma Res. 2004;14:295-299.
31. Legha SS, et al. A prospective
evaluation of a triple-drug regimen containing cisplatin, vinblastine, and
dacarbazine (CVD) for metastatic melanoma. Cancer 1989;64:20224-20229.
32. Buzaid AC, et al. Cisplatin (C),
vinblastine (V), and dacarbazine (D) (CVD) versus dacarbazine alone in
metastatic melanoma: preliminary results of a phase II cancer community
oncology group (CCOP) trial. Proc Am Soc Clin Oncol. 1993;12:389.
33. Del Prete SA, et al. Combination
chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in
metastatic melanoma. Cancer Treat Rep. 1984;68:1403-1405.
34. McClay EF, et al. Effective
combination chemo/hormonal therapy for malignant melanoma: experience with
three consecutive trials. Int J Cancer. 1992;50:553-556.
35. Rusthoven JJ, et al. Randomized,
double-blind, placebo-controlled trial comparing the response rates of
carmustine, dacarbazine, and cisplatin with and without tamoxifen in patients
with metastatic melanoma. National Cancer Institute of Canada Clinical Trials
Group. J Clin Oncol. 1996;14:2083-2090.
36. Creagan ET, et al. Phase III
clinical trial of the combination of cisplatin, dacarbazine, and carmustine
with or without tamoxifen in patients with advanced malignant melanoma. J
Clin Oncol. 1999;17:1884-1890.
37. Chapman PB, et al. Phase III
multicenter randomized trial of the Dartmouth regimen versus dacarbazine in
patients with metastatic melanoma. J Clin Oncol. 1999;17:2745-51.
38. Falkson CI. Experience with
interferon alpha 2b combined with dacarbazine in the treatment of metastatic
malignant melanoma. Med Oncol. 1995;12:35-40.
39. Young AM, et al. Prospective
randomized comparison of dacarbazine (DTIC) versus DTIC plus interferon alpha
(IFN-alpha) in metastatic melanoma. Clin Oncol (R Coll Radiol.)
2001;13:458-465.
40. Thompson DB, et al.
Interferon-alpha 2a does not improve response or survival when combined with
dacarbazine in metastatic malignant melanoma: results of a multi-institutional
Australian randomized trial. Melanoma Res. 1993;3:133-138.
41. Bajetta E, et al. Multicenter
randomized trial of dacarbazine alone or in combination with two different
doses and schedules of interferon alfa-2a in the treatment of advanced
melanoma. J Clin Oncol. 1994;12:806-811.
42. Atkins MB, et al. High-dose
recombinant interleukin 2 therapy for patients with metastatic melanoma:
Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol.
1999;17:2105-2116.
43. Kruit WH, et al. Final report of
a phase II study of interleukin 2 and interferon alpha in patients with
metastatic melanoma. Br J Cancer. 1995;71:1319-1321.
44. Marincola FM, et al. Combination
therapy with interferon alfa-2a and interleukin-2 for the treatment of
metastatic cancer. J Clin Oncol. 1995;13:1110-1122.
45. Oldham RK, et al. Combination
biotherapy utilizing interleukin-2 and alpha interferon in patients with
advanced cancer: a National Biotherapy Study Group Trial. Mol Biother.
1992;4:4-9.
46. Sparano JA, et al. Randomized
phase III trial of treatment with high-dose interleukin-2 alone or in
combination with interferon alfa-2a in patients with advanced melanoma. J
Clin Oncol. 1993;11:1969-1977.
47. Proleukin package insert.
Emeryville, CA: Chiron Therapeutics. September 2000.
48. Schrader AJ. Combined
chemoimmunotherapy in metastatic melanoma: is there a need for the double? Anti-Cancer
Drugs 2000;11:143-148.
49. Keilholz U, et al. Interferon
alfa-2a and interleukin-2 with or without cisplatin in metastatic melanoma: a
randomized trial of the European Organization for Research and Treatment of
Cancer Melanoma Cooperative Group. J Clin Oncol. 1997;15:2579-2588.
50. Eton O, et al. Sequential biochemotherapy
versus chemotherapy for metastatic melanoma: results from a phase III
randomized trial. J Clin Oncol. 2002;20:2045-2052.
51. Eleutherakis-Papaiakovou V, et
al. Thalidomide in cancer medicine. Ann Oncol. 2004;15:1151-1160.
52. Pawlak WZ, Legha SS. Phase II
study of thalidomide in patients with metastatic melanoma. Melanoma Res.
2004;14:57-62.
53. Reiriz AB, et al. Phase II study
of thalidomide in patients with metastatic melanoma. Melanoma Res.
2004;14:527-531.
54. Hwu W-J, et al. Temozolomide plus
thalidomide in patients with advanced melanoma: results of a dose-finding
trial. J Clin Oncol. 2002;20:2610-2615.
55. Hwu W-J, et al. Phase II study of
temozolomide plus thalidomide for the treatment of metastatic melanoma. J
Clin Oncol. 2003;21:3351-3356.
56. Danson S, et al. Randomized phase
II study of temozolomide given every 8 hours or daily with either interferon
alfa-2b or thalidomide in metastatic malignant melanoma. J Clin Oncol.
2003;21:2551-2557.
To comment on this article, contact
[email protected]











