US Pharm. 2006;8:HS15-HS27.
The pediatric population is a very dynamic group of patients, since the physiologic processes that determine drug disposition undergo rapid changes as children grow and mature. Therefore, the use of pharmacologic agents in pediatric patients warrants special considerations.1,2 When administering general anesthesia medications to children, a clear understanding of the physiologic, pharmacologic, and psychological differences between children and adults is an essential component of good patient care.3 This article discusses the principles and risks and complications associated with pediatric general anesthesia. Significant emphasis is placed on the various pharmacologic agents used to anesthetize pediatric patients.
Pediatric Pharmacokinetics
In comparison to adults, infants and young children respond differently to
anesthesia medications due to many factors, including body composition,
protein binding, body temperature, distribution of cardiac output, and
functional maturity of the liver and kidneys.3,4
Body components (fat, muscle, and water) change with age. Total body water is substantially higher in premature infants than in term infants and higher in term infants than in toddlers. Additionally, fat and muscle content increase with age. These changes can significantly impact the pharmacokinetics of certain medications. Consequently, water-soluble drugs that have large volumes of distribution (e.g., succinylcholine) require larger initial doses in neonates. Similarly, drugs that depend on redistribution into fat for termination of action (e.g., thiopental) have a longer clinical effect in neonates.
Neonates have decreased hepatic and renal function and protein binding, compared to older children, who generally have mature renal and hepatic function and normal adult values for protein. In addition, more of the cardiac output is diverted to the liver and kidneys in older children than in infants. Consequently, the metabolism and excretion of many drugs, such as morphine, are slowed in neonates, leading to a prolonged elimination half-life.3 Most medications have a prolonged elimination half-life in premature and term infants, a shortened half-life in children older than 2 years up to early teen years, and a lengthened half-life in teenagers approaching adulthood.3
Temperature regulation is important in pediatric anesthesia. Young children have disproportionately large body surface areas, and heat loss is exaggerated during anesthesia, especially during the induction of anesthesia, unless this is actively prevented.4 Hypothermia, particularly in neonates, delays the metabolism and excretion of anesthetic agents and can also potentiate neuromuscular blockade.5
General Anesthesia
The perioperative period (immediately before, during, and after surgery) is a
particularly critical time for pediatric patients. In general anesthesia,
medications blunt physiologic responses and render patients unaware of what is
being done to or around them. The increased risk for morbidity and mortality
in the perioperative period demands the utmost vigilance. Such risks may be
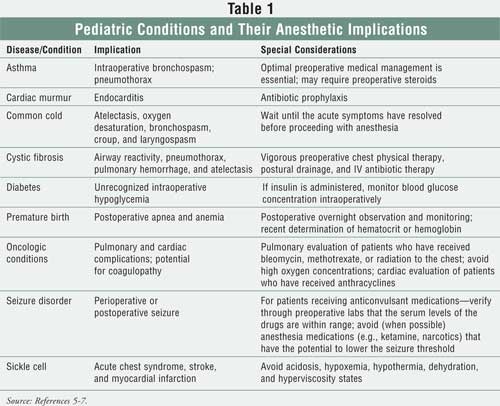
increased in certain disease states.5 Table 1 lists specific
conditions and their anesthetic complications.5-7
The purpose of general anesthesia is to suppress the conscious perception of, and physiologic response to, noxious stimuli and to render the patient unconscious. This is done through analgesia (decreasing pain), amnesia (blunting consciousness), akinesia (preventing movement), physiologic support (maintaining cardiovascular function, fluid management, electrolyte control, and thermoregulation), and vigilance.5
Prior to receiving anesthesia, all children should have a preanesthetic assessment that includes a brief history, notation of medical allergies, and a physical examination focusing on the airway, lungs, and cardiac function. In addition, a family history regarding reactions to anesthetics, drug allergies, and incidences of sudden death intraoperatively or hyperthermia postsurgery should be obtained. In children who have received general anesthesia previously, questions should be asked concerning complications.5 During this assessment, clinicians should make every attempt to ensure that the child adhered to the recommended preoperative fasting guidelines. See Table 2 for a discussion of these guidelines.5,6

Preanesthetic Phase: The primary focus of this phase is to ensure that the patient arrives in the operating room in a calm/relaxed manner, without compromised breathing and/or impaired cardiovascular status.2,5 Numerous classes of medications can be given to children (e.g., sedatives, analgesics, anticholinergics, histamine blockers) during the preanesthetic phase.2
Induction Phase: General anesthesia can be induced with either inhalation or intravenous (IV) medications. In most instances, the volatile anesthetic agents are preferred in children over IV medications because they do not require IV access. IV medications are used to induce anesthesia in children only in rare circumstances (e.g., the patient is at risk for malignant hyperthermia [MH]). However, once the child is asleep and IV access is established, children commonly receive IV anesthesia medications.5,7
Inhalation induction begins with the patient inhaling, through a face mask, a high gas flow (5 to 7 L/min of oxygen), which is usually mixed with nitrous oxide. Once a state of euphoria is reached (60 to 90 seconds), a volatile inhalation agent is typically added into the inhaled gas mixture. This combination, leading to unconsciousness within 30 to 60 seconds, allows the child to continue breathing spontaneously. 5
Maintenance Phase: This phase is the period between induction and emergence. During this time, the child should be asleep, unaware of pain, unresponsive either with motion or hemodynamic responses to painful stimuli, and homeostatically supported. Anesthesia is usually maintained with nitrous oxide, an inhalational anesthetic, and a narcotic for intraoperative analgesia. A benzodiazepine can be added to the regimen either during premedication or intraoperatively to supplement hypnosis and amnesia. Neuromuscular blockers are used when muscle paralysis is needed.5
In some circumstances, anesthesia can be maintained solely with a volatile anesthetic agent. However, this is safe only when the airway is secure and patent, the stomach is empty, the child is older than 6 months, and the duration of the procedure is less than one hour.5
Reversal Phase: The volatile inhalation agents rapidly leave the lungs during ventilation and thus do not require other products to reverse their actions. However, certain neuromuscular blockers commonly need to be reversed with an acetylcholinesterase inhibitor.5 Generally, the effects of other agents (e.g., narcotics, benzodiazepines, IV hypnotics) used in general anesthesia may be prolonged but do not usually need to be reversed.2
Volatile Inhalation Agents
Five volatile inhalation agents are commercially available in the United
States: desflurane, sevoflurane, isoflurane, enflurane, and halothane.
Desflurane, sevoflurane, and isoflurane are the most commonly used in
pediatric clinical practice.1,2 Halothane is the prototypical
pediatric inhalational agent; however, its use has decreased dramatically
since the availability of isoflurane and sevoflurane. Enflurane is rarely used
in children.5
The advantages of the volatile anesthetics include rapid onset, rapid offset, and convenient route of delivery. Additionally, the volatile anesthetics provide analgesia and amnesia. However, all of these products (in varying degrees) are airway irritants and can cause laryngospasm, breath holding, coughing, and salivation in children.5,8
All of the volatile inhalation agents depress ventilation and dilate constricted bronchial musculature. 2,5 They also commonly induce apnea and hypoxia in premature infants and newborns; thus, these anesthetics are not routinely used in children younger than 6 months. Finally, the volatile inhalation products can cause unwanted cardiovascular effects (e.g., depressed cardiac output, decreased oxygen delivery, peripheral vasodilation, cerebrovasodilation, and decreased ejection fraction) that can negatively impact anesthesia.5
Halothane has a high solubility in tissues and is therefore associated with a slow onset and termination of effect and recovery. Patients receiving halothane have an increased risk of arrhythmias and hepatotoxicity. Consequently, halothane is rarely used in pediatrics today.9 Agents that are more pleasant to breathe and that induce anesthesia more rapidly have replaced halothane in clinical practice.2
Isoflurane maintains cardiac output and cerebral perfusion better than halothane. It is also less of a respiratory depressant than halothane. Emergence from anesthesia with isoflurane is quite smooth and faster than with halothane. Isoflurane is pungent and a significant airway irritant with an unacceptably high incidence of laryngospasm; therefore, it should not be used to induce anesthesia. Because isoflurane is not a suitable induction agent, induction with sevoflurane, and maintenance with isoflurane, is a common pediatric anesthesia practice.5
Desflurane has the lowest blood gas solubility coefficient (0.42) of all of the volatile anesthetics; therefore, desflurane is associated with a fast onset and termination of effect and recovery.2 Unfortunately, desflurane (like isoflurane) is a potent airway irritant and cannot be used to induce anesthesia.5 Because desflurane has a low fat/blood solubility, it is primarily used to maintain anesthesia in longer procedures because less of the drug accumulates in the fat.2
Sevoflurane is much less pungent than isoflurane and desflurane and has a low blood gas partition coefficient, like desflurane.2 In addition, sevoflurane appears to have fewer hemodynamic effects than halothane and the profile of respiratory effects at 1 minimum alveolar concentration (MAC) appears to be similar. (See Table 3 for a discussion of MAC.) Emergence from sevoflurane is quite rapid. The major use of sevoflurane is induction of anesthesia in children. Metabolism of sevoflurane yields free fluoride, which may cause renal damage; therefore, the FDA has restricted the use of sevoflurane to less than two MAC hours, preferably with fresh gas flow rates in excess of 2 L/min.5
IV Anesthetic Agents
Anesthesia can be both induced and maintained with either boluses or
continuous infusions of IV anesthetic agents. IV anesthetic agents include
barbiturates, opioid narcotics, benzodiazepines, and miscellaneous products
(e.g., ketamine, propofol). IV anesthetic agents induce anesthesia more
rapidly than inhaled products and with fewer complications. On the other hand,
an IV line must be placed and, unless IV access is already obtained,
inhalation induction may remain the preferred route. For children arriving in
the operating room with IV access, IV induction should be routine, because it
rapidly takes the child from awake to anesthetized with less hemodynamic and
cardiorespiratory compromise.5
Barbiturates are commonly administered to pediatric patients for premedication and induction of anesthesia.10 It is important to note that the barbiturates do not provide analgesia.5 Generally, neonates require lower mg/kg doses of barbiturates than do older children; and older children require larger mg/kg doses of barbiturates than do adults.10 Thiopental, pentobarbital, and methohexital are the most frequently used barbiturates in pediatric anesthesia.3,5,10
Thiopental, the most commonly used barbiturate in pediatric anesthesia, also depresses respirations, induces apnea, and can cause hypotension in the hypovolemic patient. Thiopental generally has little impact on myocardial function and in the euvolemic, well child is a useful anesthetic induction agent. Induction with 3 to 5 mg/kg of thiopental usually produces five to 10 minutes of unconsciousness within seconds.5
Pentobarbital can also be used for sedation in children. It is a potent respiratory depressant, particularly in conjunction with narcotics and benzodiazepines. Pentobarbital is not an ideal drug for sedation for short procedures because of its prolonged duration of action.5
Methohexital is similar to thiopental and has a similar spectrum of respiratory depression to thiopental. 5 Problems associated with the IV administration of methohexital are burning, hiccups, apnea, and extrapyramidal-like movements. Consequently, IV methohexital is rarely used to induce anesthesia. Methohexital is more commonly given rectally to induce anesthesia.10
Opioids are widely used in the practice of pediatric anesthesia and pediatric perioperative med icine; they provide analgesia for painful procedures and decrease the incidence of postprocedural pain.5,11 Because opioids suppress the carbon dioxide response, induce apnea, and are respiratory depressants, their use must be monitored closely--especially when they are administered with other respiratory depressing agents (e.g., inhalational anesthetics, barbiturates, benzodiazepines).5 Morphine and fentanyl are the most commonly used opioids in pediatric anesthesia.11
Morphine is a long-acting analgesic medication that provides effective analgesia.11 Because morphine can cause bronchospasm (secondary to histamine release), it should be used cautiously in children who suffer from asthma.5 The usual IV dose of morphine is 0.1 to 0.2 mg/kg.8,11 Equivalent mg/kg doses of morphine provide much higher blood concentrations in neonates than in older children, with plasma concentrations approximating three times those in adults. This is caused by a prolonged elimination half-life (14 hours) in neonates as compared with adults (two hours).5 By 2 months of age, clearance values for morphine reach adult values.11 Because of the prolonged activity and hemodynamic instability induced by morphine, fentanyl has largely replaced morphine in general anesthesia.5
Fentanyl (which is 80 to 100 times more potent than morphine) ranks as the most commonly used opioid in pediatric anesthesia.3,5,11 It has a shorter duration of activity and a more stable hemodynamic profile than morphine.3,5 Effective analgesia and anesthesia with fentanyl can be provided using a 2- to 3-mcg/kg bolus followed by a 1- to 3-mcg/kg/hour continuous infusion.5
Other synthetic opioids--sufentanil, alfentanil, and remifentanil--are also available. Sufentanil (which is eight to 10 times more potent than fentanyl) is used primarily in pediatric cardiac cases. Remifentanil (which is similar in potency to fentanyl) is considered an ultrashort-acting opioid.5,11,12 It is used when anesthesia needs to be rapidly induced.5 Because the primary narcotic effect of remifentanil ends so quickly, additional analgesics must be used to provide postoperative pain relief.12 Alfentanil (which is five to 10 times less potent than fentanyl but has a faster onset of action) appears to have an increased incidence of muscle rigidity, convulsions, and prolonged respiratory depression compared with fentanyl and is therefore rarely used in pediatric anesthesia.5,11
Benzodiazepines induce hypnosis, anxiolysis, sedation, and amnesia and have anticonvulsant activity. In larger doses, they cause respiratory depression and apnea; they are synergistic with narcotics and barbiturates in their respiratory depressant effects. The most commonly used benzodiazepine in pediatric anesthesia is midazolam. It is a potent hypnotic-anxiolytic-anticonvulsant and is approximately four times more potent than diazepam. In anxiolytic doses, IV midazolam (0.15 mg/kg) has no impact on respiratory rate, heart rate, or blood pressure and provides excellent preoperative sedation, frequently accompanied by amnesia.5 Midazolam can be administered orally, nasally, rectally, and intramuscularly. The use of oral midazolam at a dose between 0.5 to 1 mg/kg (with a maximum of 20 mg), mixed in sweet-flavored syrup, induces anxiolysis in approximately 90% of children.5,8
Propofol has a very rapid onset and is associated with a reduced rate of postoperative nausea and vomiting. Propofol ranks as the most commonly used IV induction agent in pediatric anesthesia.2,5,13 As with thiopental, the induction dose of propofol is higher in younger patients (2.9 mg/kg for children less than 2 years of age) than in older patients (2.2 mg/kg for patients 6 to 12 years of age). Propofol is associated with pain on IV administration, particularly in small veins. As little as 0.2 mg/kg of lidocaine (mixed with the propofol) has been effective in reducing this discomfort.3 After induction of anesthesia, propofol is considered a useful agent for maintaining hypnosis and amnesia. It can be used as a sole anesthetic agent for nonpainful procedures. Combined with narcotics, propofol provides excellent, brief anesthesia for painful procedures. Propofol should not be used in children younger than 12 years of age for prolonged sedation because of the risk of serious consequences (e.g., hemodynamic collapse, metabolic acidosis, cardiac failure, profound shock, and death). Additionally, the use of propofol for prolonged sedation in the critical care setting is also not advised.5
Ketamine, which has a rapid onset and a short duration of action, causes central dissociation while providing analgesia and amnesia.3,5,10 Induction with 2 mg/kg of ketamine usually produces 15 to 30 minutes of unconsciousness within seconds.5 Ketamine may also be administered rectally at 10 mg/kg, orally at 6 to 10 mg/kg, or intranasally at 3 to 6 mg/kg to induce anesthesia.3,5 Ketamine does not significantly affect blood pressure and/or cardiac output. Because ketamine increases the production of secretions, anticholinergic medications (e.g., atropine, glycopyrrolate) are usually needed.2,5 Dreams and hallucinations are the most common side effects associated with ketamine: 5% to 10% of prepubescent children and 30% to 50% of older children experience this adverse effect.2,10 Administration of a benzodiazepine concomitantly with ketamine decreases the incidence of dreams and hallucinations.2,5 Contraindications to the use of ketamine in children include the presence of an active upper respiratory tract infection, increased intracranial pressure, open-globe injury, and the presence of psychiatric or seizure disorders.5
Neuromuscular Blocking Agents
The neuromuscular blocking agents are used primarily as an adjunct to general
anesthesia to facilitate endotracheal intubation and to maintain muscle
relaxation during surgery.2,5 It is important to note that
neuromuscular blocking agents have no known effect on consciousness or pain
threshold. Neuromuscular blocking agents are classified based on the type of
blockade produced (depolarizing versus nondepolarizing) and on their duration
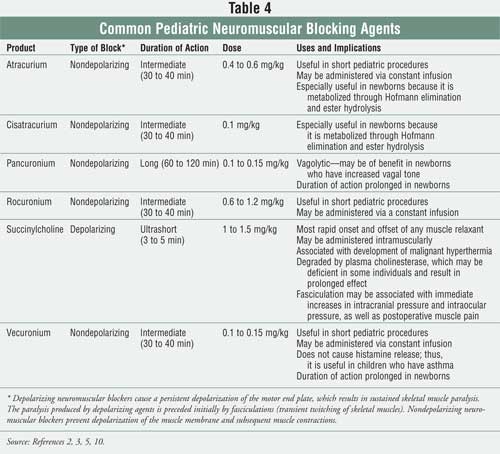
of action (e.g., ultrashort, short, intermediate, long).2 Table 4
provides information regarding the neuromuscular blocking agents that are
most commonly used in pediatrics.
The actions of the nondepolarizing neuromuscular blocking agents cease spontaneously as plasma concentrations decline or by reversal with anticholinesterase inhibitors (e.g., neostigmine). Unfortunately, the anticholinesterase inhibitors can cause unwanted cholinergic side effects, including bradycardia, bronchoconstriction, and salivation. Consequently, anticholinergic agents (e.g., atropine, glycopyrrolate) are usually given along with the anticholinesterase inhibitors in order to minimize the cholinergic side effects.2
In December 2005, the Institute for Safe Medication Practices (ISMP) issued a safety alert entitled "Paralyzed by Mistakes," which warned about inadvertently giving neuromuscular blocking agents to patients who are not receiving ventilator support. The alert noted that some patients have died or incurred permanent injuries as a result of these errors. To decrease this type of error, ISMP suggests that health care facilities provide:
• Limited access to the
neuromuscular blockers, allowing floor stock only in the operating room,
emergency department, and critical care units;
• Separate storage, keeping boxes containing these agents separate in
refrigerators and on shelves;
• Mandated warning labels on vials, syringes, infusion bags, and boxes
that state: "Warning: Paralyzing agent--causes respiratory arrest";
• A double-check process prior to administration; and
• Immediate removal of the agents once the patient has been extubated.
14
Complications Associated with
General Anesthesia
Complications associated with general anesthesia can be divided into four
basic categories: postoperative nausea and vomiting (PONV), oral trauma,
thermoregulation issues, and cardiorespiratory complications.5,6
PONV is the most common problem associated with general anesthesia. An estimated 40% to 50% of children experience PONV after receiving general anesthesia. PONV can occur in the immediate postoperative period, one to two hours postoperatively, or several hours after surgery. The stress and trauma of surgery and the proemetic effects of the anesthetic agents and narcotic analgesics most likely cause PONV.5 Numerous products exist that can prevent and/or treat PONV. The serotonin antagonists (e.g., ondansetron, dolasetron, granisetron) are considered to be first-line drugs for PONV. They can be used to treat and/or to prevent PONV. Studies show that adding dexamethasone to a serotonin antagonist further decreases the incidence of PONV. IV metoclopramide (0.1 to 0.2 mg/kg up to 10 mg) is also an effective and safe antiemetic for both prevention and treatment of PONV. The use of droperidol, which was once considered the gold standard in the treatment of PONV, has decreased significantly in recent years owing to the fact that the product, even at low doses, can cause QTc (corrected QT) interval prolongation and/or torsades de pointes.3
Oral trauma is relatively common after general anesthesia. The insertion or removal of the endotrachael tube can irritate the throat, causing a sore throat and/or hoarseness. In rare instances, dental trauma can also occur due to the insertion and/or removal of the tube.6
MH is an inherited disorder that is triggered by the volatile anesthetic agents and/or succinylcholine. It usually occurs within the first two hours of anesthesia but can occur up to 24 hours later. MH is characterized by the development of a sudden and rapid high fever (38.5°C to 46°C, rising 1°C every five minutes), muscle rigidity, acidosis, and cardiac arrhythmias. Because MH can be fatal, aggressive therapy is warranted. The treatment of MH includes discontinuing all volatile inhalation agents, correction of the metabolic acidosis, and treatment with IV dantrolene (2.5 mg/kg up to 10 mg/kg). All pharmacists, especially those working in intensive care units and operating rooms, should be familiar with reconstituting and administering IV dantrolene.
According to Donald Davis, MD, Anesthesiologist at Children's Healthcare of Atlanta, "Patients experiencing MH frequently require extremely large doses of IV dantrolene within a short period of time. This can be very challenging in terms of time and manpower since IV dantrolene can be very cumbersome to reconstitute." Davis notes that during such crisis situations, pharmacists should immediately start reconstituting IV dantrolene and continue until they are told otherwise.15 Once a person is diagnosed with MH, their relatives should be tested to determine if they also have the disorder.2,3,5
Cardiorespiratory complications (e.g., respiratory depression, hypertension, arrhythmias, heart attacks, stroke) can also result from general anesthesia. Fortunately, these problems are extremely rare and are more likely related to surgical complications. 3,5,6
Preventing Oversedation
The American
Academy of Pediatrics Committee on Drugs (COD) notes that children tend to be
particularly susceptible to oversedation with anesthesia. (Table 5
lists recommendations from the COD for preventing oversedation in children.
16) Consequently, provision for safe sedation in children requires skill
and organization of resources to prevent severe negative patient outcomes.
Pulse oximetry is considered the single most helpful monitoring device for
detecting impending life-threatening events. Pulse oximetry, particularly the
type that provides an audible change in tone as the saturation changes, should
be required for every patient sedated for a procedure, because it provides an
early warning of developing oxygen desaturation. In addition, both clinicians
administering anesthesia and those in the postoperative arena should always
have the appropriate reversal agents (e.g., naloxone, flumazenil) at their
disposal.17

Conclusion
Pharmacists should
be encouraged to take a proactive approach in managing the "littlest of
patients" during the perioperative period, especially in monitoring for side
effects and adverse drug reactions. In order to do this more effectively,
pharmacists must have an understanding of the physiologic, pharmacologic, and
psychological differences between children and adults with regard to
anesthesia pharmacology.
REFERENCES
1. Blaho K, Winbery
S, Merigian K. Pharmacological considerations for the pediatric patient.
Optom Clin. 1996;5:61-90.
2. Koda-Kimble MA,
Young LY, et al. Applied Therapeutics: The Clinical Use of Drugs. 8 ed.
Philadelphia, PA: Lippincott Williams and Wilkins; 2005.
3. Miller RD, Cucchiara
RF, et al. Anesthesia. 6th ed. Philadelphia, PA: Churchill Livingstone; 2005.
4. Motoyama EK, Davis
PJ. Smith's Anesthesia for Infants and Children. 7th ed.
Philadelphia, PA: Mosby Elsevier Science; 2006.
5. Behrman RE, Kliegman
RM, Jenson HB. Nelson Textbook of Pediatrics. 17th ed. Philadelphia,
PA: Elsevier Science; 2004.
6. American Academy of
Pediatrics. Evaluation and preparation of pediatric patients undergoing
anesthesia. Pediatrics. 1996;98(3 pt 1):502-508.
7. Ettinger AB,
Devinsky O. Managing Epilepsy and Co-existing Disorders. Boston:
Butterworth-Heinemann; 2002:155-173.
8. Taketomo CK, Hodding
JH, Kraus DM. Pediatric Dosing Handbook. 10th ed. Hudson, OH: Lexi
Comp, Inc.; 2003.
9. Golembiewski J.
Considerations in selecting an inhaled anesthetic agent: case studies. Am J
Health-Syst Pharm. 2004;61(suppl 4):S10-S17.
10. Gregory GA.
Pediatric Anesthesia. 4th ed. Philadelphia, PA: Churchill Livingstone;
2002.
11. Jablonka DH, Davis
PJ. Opiods in pediatric anesthesia. Anesthesiol Clin North America.
2005;23:621-634.
12. Hollinger I.
Current trends in pediatric anesthesia. Mt Sinai J Med. 2002;69:51-54.
13. AANA-ASA Joint
statement regarding propofol administration. April 14, 2004. Available at:
www.asahq.org/news/propofolstatement.htm.
14. Paralyzed by
mistakes: preventing errors with neuromuscular blocking agents. ISMP
Medication Safety Alert. September 22, 2005. Available at:
www.ismp.org/Newsletters/acutecare/articles/20050922.asp. Accessed April 14,
2006.
15. Interview with
Donald Davis, MD. April 14, 2006.
16. Anon. Guidelines
for monitoring and management of pediatric patients during and after sedation
for diagnostic and therapeutic procedures: Addendum. Pediatrics.
2002;110:836-838.
17. Cote CJ, Notterman
DA, et al. Adverse sedation events in pediatrics: A critical incident analysis
of contributing factors. Pediatrics. 2000;105:805-814.
To comment on this article, contact [email protected].











