US Pharm. 2007;32(4)(Oncology suppl):20-24.
Prostate cancer is the most common solid tumor affecting men in the United States. Data from 2005 estimated that approximately 232,000 men were newly diagnosed with prostate cancer and about 30,000 died from the disease.1 According to the American Cancer Society, about one third of newly diagnosed prostate cancer is considered locally advanced. At diagnosis, if the cancer is found to be locally advanced or metastatic, the standard treatment of choice is hormonal or androgen-deprivation therapy (ADT), possibly in combination with local therapy. ADT induces castrated levels of testosterone, which causes epithelial cells of the prostate gland to undergo apoptosis, and is achieved either via bilateral orchiectomy or by luteinizing hormone-releasing hormone agonist injections, such as leuprolide (Lupron, Eligard, Viadur) or goserelin (Zoladex).
Initially, most patients respond to ADT. The androgen-dependent period in patients with metastatic disease is reported to last about 14–30 months.2 The cancer then progresses to an androgen-independent stage where even castrate testosterone levels fail to control the malignancy. Eventually, the cancer no longer responds to any hormonal therapy and is then referred to as hormone-refractory prostate cancer (HRPC). HRPC can be defined as an increase in prostate-specific antigen (PSA), radiologic progression (metastases) or clinical progression (worsening pain, urinary obstruction).3 The median survival for patients after the development of metastatic HRPC ranges from nine to 12 months,4 and treatment options at this point are limited. Radiotherapy may be effective for painful bony metastasis or managing complications such as spinal core compression. Hormonal manipulations are sometimes used to decrease PSA level. Antiandrogens such as bicalutamide (Casodex), flutamide (Eulexin), and nilutamide (Nilandron) can be added, changed or withdrawn at this time. Ketoconazole plus corticosteroids can also be used to cause adrenal androgen synthesis inhibition.5 However, eventually most prostate cancer becomes refractory to these interventions. The role of chemotherapy, such as mitoxantrone, has historically been limited to palliation of bone pain. Recently, docetaxel-based therapy emerged as a new standard of chemotherapy that not only impacts bone pain and quality of life (QOL), but also shows improvement in survival.
Mitoxantrone is an anthraquinone derivative that has less cardiotoxicity than doxorubicin.6 Its usage in combination with steroids is FDA approved for palliative painful bone metastases in prostate cancer. Clinical trials with mitoxantrone have demonstrated its palliative role. A palliative study conducted with mitoxantrone (Canadian phase III study) randomized 161 hormone-refractory patients with pain to either mitoxantrone 12 mg/m2 plus prednisone 10 mg daily or prednisone alone (10 mg daily). The primary end point was palliative response, observed in 29% of patients on mitoxantrone and prednisone arm versus only 12% in patients who received prednisone alone (P = .01). The duration of palliation was also longer in the mitoxantrone and prednisone group: 43 weeks versus 18 weeks in the prednisone alone group (P <.0001). PSA decreased in the combination group, but this did not translate to improved survival. In fact, no differences in overall survival were observed.7
The Cancer and Leukemia Group B (CALGB) also showed similar results. HRPC patients were randomized to mitoxantrone 14 mg/m 2 plus hydrocortisone 40 mg (MH) or hydrocortisone 40 mg daily alone (H). Disease progression was slightly better in the MH group (3.7 months) compared to the hydrocortisone group (2.3 months) (P = .02), but again there was no difference in survival.8 Phase III trials of mitoxantrone-based chemotherapy showed an improvement in bone pain but failed to demonstrate an improvement in survival.7,8
Other chemical agents that have been studied for the treatment of prostate cancer are the taxanes. Paclitaxel (Taxol) and docetaxel (Taxotere) are the two examples in this class. They exhibit antineoplastic activity by stabilization of microtubules bundles that disrupt the equilibrium between polymerization and depolymerization.9 They also cause induction of BCL-2 phosphorylation.10 The progression of prostate cancer and the cause of the hormone-refractory state have been reported to be due to the over expression of the antiapoptotic gene BCL-2.11 In vitro, the taxanes have been shown to promote apoptotic cell death in the prostate by phosphorylating BCL-2. Docetaxel appears to be the most potent agent in BCL-2 inactivation. 12
Although there have been no randomized paclitaxel phase III studies, a phase II study conducted by the US Oncology Group randomized 163 patients to weekly paclitaxel plus estramustine phosphate (EMP) or weekly paclitaxel alone. This trial showed that PSA response was better in the paclitaxel and EMP group than in the paclitaxel group (47% versus 27%, P <.01). However, the differences in the median survivals were not significant. 13
Up to this point, chemotherapy's role in prostate cancer has been only for palliation. Not until recently has chemotherapy been used as a viable treatment option for patients with HRPC. It now can be used to decrease pain, and for the first time a chemotherapy-based regimen has been shown to also increase survival. This is due to the results of the landmark phase III clinical trials SWOG 99-16 and TAX 327, which used docetaxel-based chemotherapy.
In the SWOG 99-16 trial, EMP was used with docetaxel. EMP has both nitrogen mustard and estradiol components and is approved for use in patients with metastatic prostate cancer. It has estrogenic activity that can decrease testosterone to castrate level and it also exerts cytotoxic effects by interfering with the microtubule structure and binding to the nuclear matrix.14 EMP also has synergistic activity with other microtubule-targeting agents, such as the taxanes and vinca alkaloids, against human prostate cancer cell lines.11 When EMP was used in combination with docetaxel in phase I and II clinical trials, it produced impressive results. Although the number of patients studied was small (ranged from 30 to 46 patients), improved survival (ranged from 14 to 20 months) and a decrease in PSA ranging from 45% to 76% was shown.15-18 In October 1999, the Southwest Oncology Group (SWOG) initiated a phase III, multicenter, randomized trial comparing docetaxel and EMP with the standard chemotherapy at the time--mitoxantrone-prednisone in men with HRPC.19
In this study, a total of 770 patients with androgen-refractory prostate cancer were randomized to either docetaxel 60 mg/m 2 every three weeks (21 days) plus EMP 280 mg three times a day on days 1 to 5 or mitoxantrone 12 mg/m2 every three weeks (21 days) plus prednisone 5 mg twice a day. Antiandrogens and bisphosphonate therapy were discontinued at least one month prior to enrollment. The treatment can be titrated up to 70 mg/m2 docetaxel or 14 mg/m2 mitoxantrone depending on toxicities.
The median follow-up period was 32 months. The results demonstrated a significant improvement in the median survival in the docetaxel-EMP group compared to the mitoxantrone-prednisone group (17.5 months vs. 15.6 months) (TABLE 1). This translates to a death reduction of 20% in the docetaxel group. Better time-to-disease progression (6.3 months vs. 3.2 months), which is defined as worsening lesion on bone scan and a better PSA response, was seen in the docetaxel and EMP group compared to the mitoxantrone and prednisone group. Response of measurable disease and subjective pain relief rates were not statistically different. Significantly more patients treated with docetaxel-estramustine had a greater than 50% decrease in PSA. Further analysis showed that survival benefit was achieved in patients in the docetaxel-EMP group in the absence of a 50% PSA decrease.

There were significantly greater toxicity rates observed in the docetaxel-EMP group compared to the mitoxantrone-prednisone group: gastrointestinal (20% vs. 5%), neutropenic fever (5% vs. 2%), cardiovascular (15% vs. 7%), metabolic disturbances (6% vs. 1%), and neurologic events (7% vs. 2%). Discontinued treatment rate was 16% in the docetaxel-EMP group compared to 10% in the mitoxantrone-prednisone group due to these adverse events. The study protocol was amended in 2001 to include the addition of oral coumadin 2 mg a day and aspirin 325 mg a day in the docetaxel-EMP group to prevent thromboembolic events.
TAX 327 was another phase III, randomized, international trial looking at survival in men with HRPC treated with docetaxel or mitoxantrone. One thousand and six patients with HRPC were randomized to three groups: docetaxel 30 mg/m2 weekly for five consecutive weeks of a six-week cycle, docetaxel 75 mg/m2 every three weeks or mitoxantrone 12 mg/m2 every three weeks.20 The two docetaxel regimens were designed to provide the same drug dose intensity over a six-week period. Docetaxel weekly was studied since it was associated with less hematologic toxicity compared with the conventional every-three-weeks schedule. All groups received prednisone 5 mg twice a day.
Median follow up was for 21 months. The survival benefit was significantly better in the every-three-week docetaxel group compared to mitoxantrone (TABLE 2), but not in the weekly docetaxel group relative to the mitoxantrone group. The median survival rates were 18.9, 17.4, and 16.5 months for triweekly docetaxel-prednisone, weekly docetaxel-prednisone, and mitoxantrone-prednisone group, respectively. This translates into a 24% reduction in the risk of death for patients in the triweekly docetaxel-prednisone group compared to the mitoxantrone-prednisone group. Another secondary end point result found that significantly more patients in the every-three-weeks docetaxel group experienced a reduction in pain compared to the mitoxantrone group. Patients in the weekly docetaxel arm also had a reduction in pain (31%), but the difference was not significant relative to mitoxantrone (P = .08). PSA response was significantly better in both docetaxel groups compared to the mitoxantrone group: 45% in every-three-weeks administration and 48% in the weekly administration arm versus 32% in mitoxantrone (P<.001). This is an interesting result, also found in the SWOG 9916 trial, because the improvement in PSA response does not seem to correlate with survival. Nearly half of the patients in the weekly docetaxel arm had a PSA response. Again, this did not translate to improved survival relative to the control arm. QOL was also significantly better in both docetaxel groups. Compared to patients in the mitoxantrone arm, 22% of patients in the every-three-week and 23% in the weekly docetaxel arms had a significant improvement in QOL.

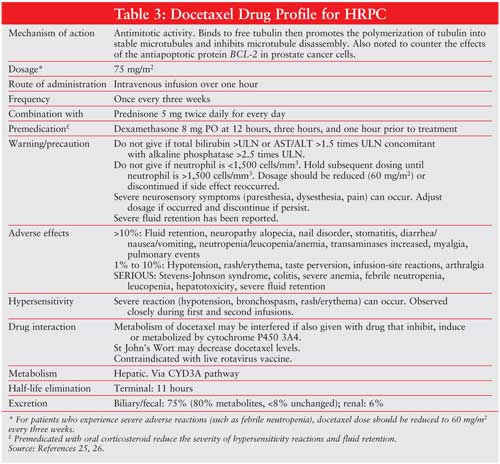
Adverse events in this trial were more common in the docetaxel arm. Grade 3–4 neutropenia occurred in 32% of patients on triweekly docetaxel, 22% of patients in the mitoxantrone group, and 1.5% of patients treated weekly with docetaxel. Febrile neutropenia and infection was uncommon in all groups. The rate of impaired left ventricular ejection fraction was significantly higher in the mitoxantrone arm (22%) than in the triweekly docetaxel (10%) and the weekly docetaxel (8%) arms. Weekly docetaxel was reported to have more clinical side effects (gastrointestinal, tearing, and epistaxis) than the triweekly dose. (SeeTABLE 3 for a detailed docetaxel profile.)
Conclusion
The role of chemotherapy in advanced prostate cancer has previously been for
palliative care without any survival benefit. This concept has changed with
recent clinical docetaxel trials. In TAX 327, docetaxel every three weeks plus
low-dose prednisone resulted in better survival improvement (2.4 months
improved), decreased pain, better PSA response, and better toxicity profile,
resulting in better QOL. In the SWOG trial, patients treated with docetaxel
and EMP showed a median overall survival prolonged by two months and a median
progression-free survival prolonged by three months. Looking at
the side effects profile, EMP-based therapy with docetaxel resulted in
gastrointestinal and cardiovascular toxicity (deep venous thrombosis and
pulmonary thromboembolism). The SWOG 9916 trial was amended to include
anticoagulation therapy (aspirin and warfarin), but the outcome cannot be
interpreted since this was not in the primary design of the study. At this
time, an EMP-based regimen should not be used since there is no further
benefit of increasing survival compared to docetaxel-based treatment alone.
Treatment of patients with HRPC is not based on any global algorithm, but should be based on specific patient cases. Things to consider are the performance status and expectations of the patient, goals of the treatment, and potential toxicity. Many questions regarding chemotherapy still remain, and time to initiate chemotherapy is still controversial. Should it be initiated in symptomatic versus asymptomatic patients? Does docetaxel have a role in earlier stages of advanced prostate cancer?
Current randomized trials should answer some of these questions. Although results from these trials are impressive, the docetaxel-based regimen still only showed improved survival of 2–2.4 months. The new era of docetaxel therapy has spurred other phase II clinical trials combining docetaxel with carboplatin,21 thalidomide,22 calcitriol,23 and bevacizumab,24 which have promising survival results and will hopefully integrate chemotherapy as part of the standard of care. What is known at this time is that the use of docetaxel every three weeks plus low-dose prednisone is the standard chemotherapy treatment for patients with HRPC.
References
1. Jenal A, Murray T, Ward E, et al. Cancer Statistics, 2005. CA Cancer J
Clin. 2005;55:10-30.
2. Sharifi N, Gulley JL, Duhut WL. Androgen deprivation therapy for prostate
cancer. JAMA. 2005;294:238-244.
3. Sonpavde G, Hutson T. New approaches in hormone refractory prostate cancer.
Am J Clin Oncol. 2006;29:196-201.
4. Ryan CJ, Small EJ. Advances in prostate cancer. Curr Opin Oncol.
2004;16:242-246.
5. Sonpavde G, Hutson T, Berry W. Hormone refractory prostate cancer:
management and advances. Cancer Treatment Reviews. 2006;32:90-100.
6. Gulley J, Dahut W. Chemotherapy for prostate cancer. Am J Therapeutics
. 2004;11:288-294.
7. Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone
plus prednisone or prednisone alone for symptomatic hormone-resistant prostate
cancer: a Canadian randomized trial with palliative end point. J Clin Oncol
. 1996;14:1756-1764.
8. Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without
mitoxantrone in men with hormorne refractory prostate cancer: result of the
cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17:2506-2513.
9. Rowinsky EK, Donehower RC. The clinical pharmacology and use of
antimicrotubule agents in cancer chemotherapeutics. Pharmacol Ther.
1991;52:35-84.
10. Silvestris N, Leone B, Numico G et al. Present status and perspectives in
the treatment of hormone-refractory prostate cancer. Oncology.
2005;69:273-282.
11. Petrylak P. Daniel. Future directions in the treatment of
androgen-independent prostate cancer. Urology. 2005;65:8-12.
12. Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity.
Cancer Res. 1997;57:229-233.
13. Berry W, Hathorn JW, Dakhil SR, et al. Phase II randomized trial of weekly
paclitaxel with or without estramustine phosphate in progressive, metastatic,
hormone-refractory prostate cancer. Clin Prostate Cancer.
2004;3:104-111.
14. Hartleyasp B, Kruse E. Nuclear-protein matrix as a target for
estramustine-induce cell-death. Prostate. 1986;9:1457-1465.
15. Petrylak DP, Shelton GB, England-Owen C, et al. Response and preliminary
survival result of a phase II study of docetaxel (D) + estramustine (E) in
patients with androgen-independent prostate cancer. Proc Am Soc Clin Oncol
. 2000;19:334a.
16. Savarese DM, Halabi S, Hars V, et al. Phase II study of docetaxel,
estramustine and low-dose hydrocortisone in men with hormone-refractory
prostate cancer: a final report of CALGB 9780. J Clin Oncol.
2001;19:2509-2516.
17. Sinibaldi VJ, Canducci MA, Moore-Cooper S, et al. Phase II evaluation of
docetaxel plus one-day oral estramustine phosphate in the treatment of
patients with androgen independent prostate cancer. Cancer.
2002;94:1457-1465.
18. Sitka Corpur M, Ledakis P, Lynch J, et al. Weekly docetaxel and
estramustine in patients with hormone-refractory prostate cancer. Semin
Oncol. 2001;28(suppl 15):16-21.
19. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine
compared with mitoxantrone and prednisone for advanced refractory prostate
cancer. N Engl J Med. 2004;351:1513-1520.
20. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med
. 2004;351:1502-1512.
21. Oh WK, Halabi S, Kelly WK, et al. A phase II study of estramustine,
docetaxel and carboplatin with granulocyte-colony-stumulating factor support
in patients with hormone-refractory prostate carcinoma-cancer and leukemia
group B 99813. Cancer. 2003;98:2592-2598.
22. Dahut WL, Arlen PM, Gulley J, et al. A randomized phase II trial of
docetaxel plus thalidomide in androgen-independent prostate cancer. Pro Am
Soc Clin Oncol. 2002;21:183.
23. Beer TM, Eilers KM, Carzotto M, et al. Weekly high-dose calcitriol and
docetaxel in metastatic androgen-independent prostate cancer. J Clin Oncol
. 2003;21:123-128.
24. Picus J, Halabi S, Rini BI, et al. The use of bevacizumab with docetaxel
and estramustine in hormone refractory prostate cancer (HRPC): initial result
of CALGB 90006. Pro Am Soc Clin Oncol. 2003;22:1578A.
25. McKeage K, Keam SJ. Docetaxel: in hormone-refractory metastatic prostate
cancer. Drugs. 2005;65:2287-2294.
26. Aventis Pharmaceuticals Inc. Taxotere (docetaxel) injections concentrated:
prescribing information 2005.
To comment on this article, contact [email protected].











