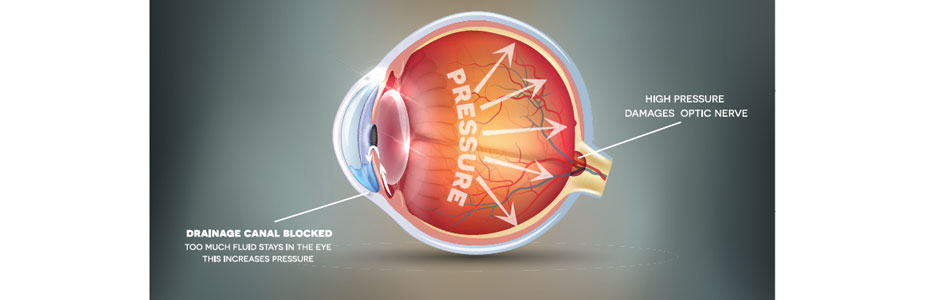
Glaucoma can be a devastating disease when left untreated. Increased intra-ocular pressure can cause a loss of peripheral vision that can be life changing. Luckily, there are excellent and simple treatments available, even in the most severe cases. The latest and greatest treatments include an eye drop called Simbrinza and a surgically placed stint called I Stent.
 Simbrinza is a combination drop of Brinzolamide 1% (Azopt) and Brimonidine tartrate 0.2% (Alphagan). It is the first combination drop that does not include a beta-blocker. When beta-blockers are used, it is a requirement to make sure the patient being treated has a current heart evaluation by their primary care doctor. Beta –blockers are drugs that slow the heart rate. A patient may already be on a prescribed by mouth beta- blocker for a diagnosed heart problem or may have an undiagnosed heart issue. Beta blockers are contraindicated for anyone with a previous myocardial infarction, a history of arrhythmia or bradycardia, pulmonary problems such as chronic obstructive pulmonary disease, or emphysema. These conditions may rule out this treatment option for some patients.
Simbrinza is a combination drop of Brinzolamide 1% (Azopt) and Brimonidine tartrate 0.2% (Alphagan). It is the first combination drop that does not include a beta-blocker. When beta-blockers are used, it is a requirement to make sure the patient being treated has a current heart evaluation by their primary care doctor. Beta –blockers are drugs that slow the heart rate. A patient may already be on a prescribed by mouth beta- blocker for a diagnosed heart problem or may have an undiagnosed heart issue. Beta blockers are contraindicated for anyone with a previous myocardial infarction, a history of arrhythmia or bradycardia, pulmonary problems such as chronic obstructive pulmonary disease, or emphysema. These conditions may rule out this treatment option for some patients.
Simbrinza is effective because it helps treat two different areas that contribute to the increase of intraocular fluid buildup. Brinzolamide is a carbonic anhydrase inhibitor. It is used suppress the activity of carbonic anhydrase. This means the decreasing of intraocular fluid. Brimonidine Tartrate 0.2% is an alpha-adrengic receptor agonist. This also reduces the production of aqueous humor but also increases uveo-scleral outflow.
The most common side effects reported with Simbrinza use were blurry vision, eye irritation, bad taste after use, dry mouth, and some eye allergy (itching, burning, etc.) This was only reported by 3-5% of all patients treated.
Simbrinza has an effective reported rate of 21- 35% decrease in intra-ocular pressure compared to other glaucoma treatments at an average of 20% decrease.
If a patient has liver problems, it may be impossible to use Simbrinza due to the brimonidine tartrate. This is a drug that is metabolized primarily by the liver. However, urinary excretion is the major route of the elimination of the drug. If a patient has corneal problems, this may also contraindicate the use of Simbrinza. Carbonic anhydrase (Brinzolamide) has been found to cause problems in both cytoplasms and around the plasma membranes of the corneal endothelium. There is an increased potential for developing corneal edema in patients with low endothelial cell counts.
Simbrinza is used three times per day. Cost is approximately $135.00 per bottle, per month. There are no generics, now. Insurances may not cover this medication.
 The I-Stent is a trabecular meshwork bypass. The device improves your eye’s natural fluid outflow to safely lower eye pressure by creating a permanent opening in the trabecular meshwork. I-Stent is the smallest medical device ever approved by the FDA. It is placed in your eye during cataract surgery and is so small that you won’t be able to see or feel it after the procedure is over. I-Stent is designed to create a permanent opening in your trabecular meshwork and works continuously to improve the outflow of fluid from your eyes to help control eye pressure. For patients with combined cataract and open-angle glaucoma, I-Stent reduces intraocular pressure (IOP) by improving aqueous humor outflow. Inserted through a 1.5-mm corneal incision, I-Stent is the only FDA-approved device for the treatment of mild to moderate open-angle glaucoma.
The I-Stent is a trabecular meshwork bypass. The device improves your eye’s natural fluid outflow to safely lower eye pressure by creating a permanent opening in the trabecular meshwork. I-Stent is the smallest medical device ever approved by the FDA. It is placed in your eye during cataract surgery and is so small that you won’t be able to see or feel it after the procedure is over. I-Stent is designed to create a permanent opening in your trabecular meshwork and works continuously to improve the outflow of fluid from your eyes to help control eye pressure. For patients with combined cataract and open-angle glaucoma, I-Stent reduces intraocular pressure (IOP) by improving aqueous humor outflow. Inserted through a 1.5-mm corneal incision, I-Stent is the only FDA-approved device for the treatment of mild to moderate open-angle glaucoma.
These two treatments have been quite successful. It is better to be pro-active with glaucoma treatments, rather than waiting till damage has already been done. Once the nerve is damaged, it is not possible to repair the damage. These treatments allow for either the ease of just using an eye drop, or even having a stint inserted during a surgical procedure such as cataract surgery that a patient would be having regardless.
The continual research and development efforts of glaucoma treatments are outstanding. This leads to an improved ability to care for patients, and an easier regimen for patients and their families to maintain.

Linda Hardy received the American Optometric Association Paraoptometric of the Year Award at the 2016 SECO meeting in Atlanta, Georgia. Linda is a Certified Optometric Technician (COT) and Certified Ophthalmic Assistant (COA). She is also ABO and NCLE certified and a Licensed Dispensing Optician (LDO) in the state of Georgia.
A graduate of Georgia Medical Institute as a Registered/ Certified Medical Assistant, Linda began her optical career working with a group of ophthalmologists as an ophthalmic assistant. She has been the Clinical Coordinator with a private optometry practice in Newnan, Georgia for 15 years.
Linda is also a speaker and educator at regional optical meetings. She and her husband have five children, and she enjoys spending time with her family, exercising, reading and volunteering in her community.













