Airport security forces have begun to experiment with computers that use pattern recognition to identify someone who's up to no good by watching for a suspicious pattern in a video image—such as an unattended bag sitting in the terminal. Interestingly, our bodies have been employing pattern recognition for security for thousands of years in the form of toll-like receptors. TLRs try to discriminate between "pathogen" and "self" by recognizing molecular patterns on the surfaces of pathogens. TLRs can then trigger either a Th1 immune response or a Th2 allergic response depending on how they are stimulated. This article will explore what we know about TLRs and speculate on how TLRs and the molecules that trigger them might be used as effective therapies.
TLRs in Action
Toll-like receptors are arguably the best characterized pattern recognition receptors, and they exist everywhere in the body.1 In the eye, TLRs can be found on the surfaces of various ocular cell types, including corneal epithelial, conjunctival epithelial, stromal fibroblast and retinal pigment epithelial cells.2 The primary function of a TLR is to recognize common components of invading pathogens referred to as pathogen-associated molecular patterns (PAMPs), which act as ligands for the TLR. Ligands are molecules that have the ability to bond to a receptor and cause a biological change. In the case of TLRs, certain ligands bind to TLRs to set off different signaling pathways. Toll-like receptors play a pivotal role in activating allergic hypersensitivity reactions and innate immunity, and in developing adaptive immunity against pathogens. They coordinate host responses by synthesizing inflammatory mediators and cytokines.3
Once a TLR recognizes a PAMP, it triggers a certain signaling cascade depending on the nature of the pathogen. Stimulation of some TLRs (chiefly TLRs 3, 7, 8 and 9) by their ligands induces a Th1 lymphocyte response—an immune response usually reserved for bacteria and viruses—whereas stimulation of other TLRs (such as TLRs 2, 4 and 5) induces a Th2 lymphocyte response, an allergic response.4 In ocular allergy, allergens that enter the tear film will push through gap junction proteins and ultimately cross-link with IgE antibodies on the surface of conjunctival mast cells. Mast cell degranulation and subsequent release of allergic and inflammatory mediators result in the cardinal signs and symptoms of itching, redness, chemosis, tearing and lid swelling.5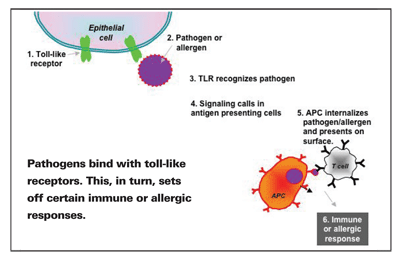
Allergic disorders have both a genetic and environmental component. Certain individuals have Th1 tendencies and others have Th2 tendencies, especially those with a predisposition to atopy. However, environmental influences may also play a key role in shifting the Th1/Th2 balance. In the germ-free living conditions that have become the norm in many Western cities, there is a lack of Th1-inducing factors that can be recognized by TLRs. This reduction corresponds with an increase in Th2-mediated disorders in the urban population which aren't prominent in the rural population. This led to the the hygiene hypothesis—removing the natural barrage of Th1 triggers has shifted the system towards Th2.6,7
TLR Breakdown
The TLR family of receptors links the extracellular compartment (contact and recognition of PAMPs) and intracellular compartment (signaling cascades occur here, which lead to cellular responses). The cytoplasmic domain resembles an IL-1 receptor (IL-1R). It's called the Toll/IL-1R (TIR) domain, and it plays a critical role in host defense and inflammation.8 TLRs have different patterns of expression (some at the cell surface, others intracellularly) depending on the nature of their ligands. In humans, 10 TLRs have been classified, whereas 13 have been recognized in mice.9
One of the first mammalian receptors to be identified was TLR4, which is the most extensively studied of all the toll-like receptors. TLR4 recognizes lipopolysaccharide (LPS), a molecule that exists on the surface of gram negative bacteria. Peptidoglycans and lipopeptides, molecules found within the cell wall of gram positive bacteria, are detected by TLR2 and its relatives TLR1 and TLR6. TLR3 is necessary for responding to double-stranded RNA (a characteristic of some viruses), TLRs 7 and 8 are responsible for recognizing single-stranded RNA, and TLR9 recognizes other types of bacterial and viral DNA motifs. TLR5 senses proteins like bacterial flagellin. Little is known about TLR10.10
TLR Signaling Pathways
Following TLR pattern recognition, signaling events induce expression of cytokines, chemokines (TNF-a, IL-6, IL-8, IL-18, MIP-1) and adhesion molecules (ICAM-1, E-selectin), which result in the activation of antigen-specific and nonspecific immune responses. Different TLR ligands wield separate (but sometimes overlapping) genetic effects due to activation of distinct and common signaling pathways leading to induction of distinct and common sets of genes.11
The steps that lead to the development of either an immune response or an allergic response depend on the balance between Th1 and Th2 lymphocytes. TLRs activate antigen presenting cells (APCs). An APC is a cell that internalizes an antigen and then displays part of it on its surface. T cells recognize the antigen and interact with it, triggering the production of more T cells and subsequent events. So, enhancing the expression of surface molecules means that with more APCs, there are more antigens being presented, and more T cells being produced (Th2 in the case of allergy), and thus an exacerbated reaction.
CD4+ T cells differentiate into Th1 or Th2 cells. Th1 cells, an immune response, produce interferon-gamma and mediate antiviral or antibacterial immunity. Most TLR ligands stimulate APCs to produce Th1-inducing cytokines and to produce Th1-biased immune responses.12
On the other hand, TLR 2 and 5 agonists can provoke Th2, or allergic, responses.12 Th2 cells secrete IL-4 and IL-13 or both, and are responsible for perpetuating the allergic response. In atopic conditions, release of histamine from mast cells increases the number of Th2 cells and reduces the number of Th1 cells.13 This imbalance created by histamine maintains an environment sensitized to the allergic response. Recent research has indicated that LPS (a ligand for TLR4) has been found to have effects that vary based on its amount.4 LPS promotes IgE synthesis in an in vitro mouse model; paradoxically, in vivo it has been shown to actually inhibit the development of allergen-specific IgE and asthma.14 Essentially, research has found that low doses of LPS lead to Th2-biased responses, whereas high doses lead to Th1 responses.4
Better Treatment through TLRs
We've begun to apply our understanding of TLRs to the development of disease therapies, including treatment for allergic hypersensitivities. Blocking or augmenting TLR's pattern recognition and defense function can permit modification of the Th1/Th2 balance, resulting in the ability to manipulate disease pathways. In addition to altering this balance, controlling the actual generation of regulatory T cells may alleviate the inflammation associated with allergy.
Researchers first described the expression patterns of TLRs in healthy and chronically allergic conjunctivae (in vernal keratoconjunctivitis patients) in 2005.15 Compared to healthy subjects, VKC conjunctivae showed a slight reduction of TLR2 expression, significant upregulation of TLR4 and downregulation of TLR9. The expression of TLR4 was localized to mast cells, which is in line with previous reports indicating that activation of TLR4 induces mast cell degranulation and release of Th2 cytokines.16
Another study found that stimulating TLR2 in a mouse model of allergic conjunctivitis suppresses eosinophil infiltration by inducing CD4+ T cell apoptosis rather than upregulating Th1 responses.17 The downregulation of TLR9 in the 2005 study on TLR expression patterns in VKC, however, contradicts other reports. In fact, TLR9 signaling activated by CpG DNA has been shown to have the strongest ability to induce Th1 cell differentiation. CpG DNA, also called CpG islands, are special sections of DNA that are usually located in areas that are essential for cell function.18
In one study, CpG DNA (a ligand for TLR9) inhibited airway eosinophilia and airway hypersensitivity and shifted cytokine balances to the Th1 side.19 CpG DNA has been demonstrated to have more powerful and long-lasting effects when conjugated with antigen (having the antigen attached to it, thereby conferring the immunological attributes of the carrier to the attached antigen), which also leads to a diminished ability of antigen to react with IgE. The release of histamine induced by antigen-CpG DNA conjugates was also much lower than that induced by antigen alone.20,21 These results indicate that conjugation also helps to reduce the allergenicity of invading antigens.
It remains unknown how CpG DNA combats allergic hypersensitivities, but there are various possibilities. It can activate antigen presenting cells to support Th1 cell stimulation and inhibit Th2 cell activation by preventing APCs from presenting allergens to the Th2 cells. CpG DNA also inhibits IgE-dependent release of Th2 cytokines, mainly IL-4, from mast cells. In addition, CpG DNA may directly act on B cells to inhibit IgE production.12 We hope that exploration of the exact mechanisms by which CpG DNA interacts with TLR9 may provide a new way to treat allergy.
Another important TLR9 ligand is ISS-ODN (synthetic immunostimulatory sequence oligodeoxynucleotide), which stimulates an innate response controlled by cytokines that are known to inhibit the allergic phenotype. ISS-ODN also downregulates IL-4 expression and IL-4 responsiveness.4 When given as an allergen-independent therapeutic agent to Th2-sensitized mice (within hours of allergen challenge), ISS-ODN has been found to reduce hypersensitivity responses associated with asthma, allergic conjunctivitis and allergic rhinitis. In addition, adaptive responses induced by allergen/ISS-ODN vaccination can mature over a period of a few weeks to be allergen specific and to imprint on memory lymphocytes and influence subsequent responses.4
Allergen ISS-ODN conjugates, known as AICs, were introduced about 10 years ago. The first one to enter clinical trials was a ragweed-specific AIC called Amb A 1.4 Conjugation was shown to cause allergens to become resistant to binding by allergen-specific IgE.22 In one trial, after six weekly injections of Amb A 1, cytokine production shifted from Th1 to Th2.23 Patients also demonstrated decreased responses to nasal allergen challenge after ragweed season and also several months following the final AIC injection.24 Although these patients did not have significant improvement immediately after the injections, there was a significant improvement in symptoms in the next allergy season. Researchers still need to perform safety evaluations, determine optimal dosing concentrations and regimens and analyze efficacy further; yet, AIC and other ISS-ODN-based interventions remain a promising option as treatments for the reversal of allergic hypersensitivities.
Other research has looked at the ability of TLR2 and TLR5 agonists to stimulate Th2 immune responses in certain conditions. While research has demonstrated that certain TLR2 ligands promote Th2 development, other reports have noted that certain TLR2 ligands inhibit Th2 cytokine responses.12
As mentioned earlier, LPS (a ligand for TLR4) can induce Th2 responses at low doses or when inhaled, which could be promising for treatment. Research has linked TLR4 mutation to susceptibility to food allergy.25 Signaling within the normal flora of the gut by way of TLR4 has been shown to play a role in inhibiting allergic hypersensitivity to food allergens.
Various microbial products have been effective for the treatment and/or prevention of allergy. In addition to CpG DNA, both pre-clinical and clinical studies have found that certain types of bacteria, particularly Lactobacilli, can act as "probiotics" that decrease inflammation associated with allergic reactions.25
Future Therapies
TLR ligands, particularly nucleic acids, can be synthesized in vitro and used as anti-allergic vaccines. If the ragweed treatment Amb A 1 AIC succeeds, this would be a therapeutic milestone, as any allergen could become more immunogenic and less allergenic. Other nucleic acid derivatives could also be used for formulating new immunotherapy techniques or be incorporated into treatments for allergic hypersensitivities (as they steer adaptive immunity away from the Th2-bias). TLR2, 4, and 5 ligands seem to be less promising for allergic disease therapy because of their Th2 preference, though.
Ultimately, an improved understanding of TLRs in relation to ocular allergy may lead to new immunotherapies that will enable us to reverse allergic hypersensitivities.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002;20:197-216.
2. Kumar A, Yu FX. Toll-like receptors and corneal innate immunity. Curr Molecular Med 2006;6:327-337.
3. Medzhitov R, Janeway C Jr. The toll receptor family and microbial recognition. Trends Microbiol 2000;8:452-456.
4. Horner AA. Update on toll-like receptor ligands and allergy: Implications for immunotherapy. Curr Allergy Asthma Rep 2006;6:395-401.
5. Abelson MB, Smith L, Chapin M. Ocular allergic disease: mechanisms, disease sub-types, treatment. Ocular Surface 2003;1:127-149.
6. Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: Revisiting the hygiene hypothesis. Nat Rev Immunol 2001;1:69.
7. Holla AD, Roy SR, Liu AH. Endotoxin, atopy and asthma. Curr Opin Allergy Clin Immunol 2002;2:141.
9. Dunne A,
10. Yu FX, Hazlett LD. Toll-like receptors and the eye. Invest Ophthalmol Vis Sci 2006;47:1255-1263.
11. Akira S. Toll-like receptor signaling. Nat Rev Immunol 2004;4:499.
12. Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immnol 2006;117:979-987.
13. Packard KA, Khan MM. Effects of histamine on Th1/Th2 cytokine balance. Int Immunopharmacol 2003;3:909-20.
14. Tulic MK, Wale JL, Holt PG, Sly PD. Modification of the inflammatory response to allergen challenge after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol 2000;22:604-612.
15. Bonini S, Micera A, Iovieno A, Lambiase A, Bonini S. Expression of toll-like receptors in healthy and allergic conjunctiva. Ophthalmology 2005;112:1528.
16. Varadaradjalou S, Feger F, Theiblemont N, et al. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol 2003;33:899.
17. Fukushima A, Yamaguchi T, Ishida W, Fukata K, Ueno H. TLR2 agonist ameliorates murine experimental allergic conjunctivitis by inducing CD4 positive T-cell apoptosis rather than by affecting the Th1/Th2 balance. Biochem Biophys Res Commun 2006;339:1048-55.
18. Kim SK, Ragupathi G, Musselli C, Choi SJ, Park YS, Livingston
19. Sur S, Wild JS, Choudhury BK, Sur N, Alam R, Klinman DM. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol 1999;162:6284-93.
20. Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides. J Immunol 2000;164:5575-82.
21. Tighe H, Takabayashi K, Schwartz D, et al. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J Allergy Clin Immunol 2000;106:124-34.
22. Horner AA, Takabayashi K, Beck L, et al. Optimized conjugation ratios lead to allergen immunostimulatory oligodeoxynucleotide conjugates with retained immunogenicity and minimal anaphylactogenicity. J Allergy Clin Immunol 2002;110:413-420.
23. Simons FE, Shikishima Y, Van Nest G, et al. Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. J Allergy Clin Immunol 2004;113:1144-1151.
24. Tulic MK, Fiset
25. Bashir MEH, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol 2004;172:6978-6987.











