Intraocular pressure is a tricky BIO- metric to manage. Pressure can fluctuate 5 to 8 points on its own in a glaucoma patient, and that may be the range of reduction in IOP we're looking for when we start therapy. If the target pressure can occur in the natural history of the disease, it's quite a challenge to figure out if our treatment has actually had an effect.
In an effort to solve this problem, ophthalmologists have adopted a strategy referred to as a monocular drug trial. This is an attempt to reveal the impact of a treatment by applying it first to one eye only and then comparing the result to the other untreated eye. In theory, any change in the untreated eye is the result of spontaneous fluctuation, while the change in the treated eye is the sum of spontaneous fluctuation and pressure reduction caused by the treatment.
For example, suppose a patient's untreated pressure is 25 mmHg in both eyes. If we treat one eye and the treated eye had a pressure of 19 at the next visit while the fellow eye was still at 25, that could be taken to mean that the treatment was effective. This approach seems very reasonable—enough so that the
However, the validity of this method depends on a series of assumptions that may or may not hold up under careful scrutiny:
1) Fellow eyes exhibit symmetrical spontaneous IOP fluctuations.
2) Fellow eyes respond symmetrically to a given medication.
3) There is no contralateral crossover effect from monocular treatment with a topical IOP-lowering agent.
4) The patient is using the medication as prescribed.
5) A change in range can be determined with a single measurement.
I believe there is compelling evidence that the assumptions underlying this approach are not necessarily valid, leaving open the possibility that in some cases—perhaps many cases—a monocular drug trial won't actually tell you whether or not your treatment worked.
The Problem of Asymmetric Shift
The primary assumption behind a monocular drug trial is that the spontaneous fluctuation that occurs in fellow eyes is symmetric, so that one eye tells you what the other eye would have done if you hadn't intervened. As it turns out, there's very little evidence in the literature to support symmetric fluctuation, and a growing body of evidence suggesting that it may not be the case.
For example:
• A 1964 study compared diurnal curve shapes between fellow eyes of 236 glaucoma patients; 45 percent of the subjects had different IOP curves in their fellow eyes.1 This phenomenon was confirmed in a 1993 study that found that 33 percent of ocular hypertensive patients and 36 percent of glaucoma patients exhibited different IOP curves between fellow eyes.2
• Recent studies by Arthur Sit, John Liu and Robert Weinreb3,4 found that while variations between the left and right eyes of subjects over a 24-hour period were similar, the strength of association between them was only moderate. (This was true for both healthy subjects and those with untreated open-angle glaucoma.)
• A study I conducted with Laurie Barber and Diana Burton5 compared the frequency and magnitude of asymmetric fluctuations of IOP between fellow eyes of glaucoma patients and normal subjects. We found that spontaneous asymmetric fluctuations of IOP between fellow eyes occur often in half of normal subjects and the majority of glaucoma patients. During those asymmetric fluctuations, IOP changed by 3 mmHg or more in one eye relative to the change in the fellow eye from one visit to the next.
• A more recent study of ours involving 17 normal subjects (not yet published) found that only 36 percent of the IOP fluctuation in a given eye could be explained by IOP fluctuation in the fellow eye.
Given that glaucoma is commonly asymmetric, I don't believe it's reasonable to expect symmetry in the pressure changes associated with it. In fact, the clinical data indicates that IOP change doesn't correlate perfectly between right and left eyes in normal subjects, so assuming symmetry in the behavior of IOP between fellow eyes in glaucoma seems unwarranted.
Medication Effects
The monocular drug trial also assumes that eyes will respond symmetrically to a given drug under similar conditions, and that the second eye is not being affected by the first eye's treatment.
The data regarding the first of these two assumptions is mixed. In one study, our group found a high correlation of IOP reduction between fellow eyes treated with the same medication (r2=0.7).6 But in a later study involving 52 patients, when we investigated how well the results of a monocular trial predicted the IOP response when a fellow eye was treated, we found no correlation (r2=0.0174) between IOP reduction in the first eye versus the second eye.7 (See chart below.)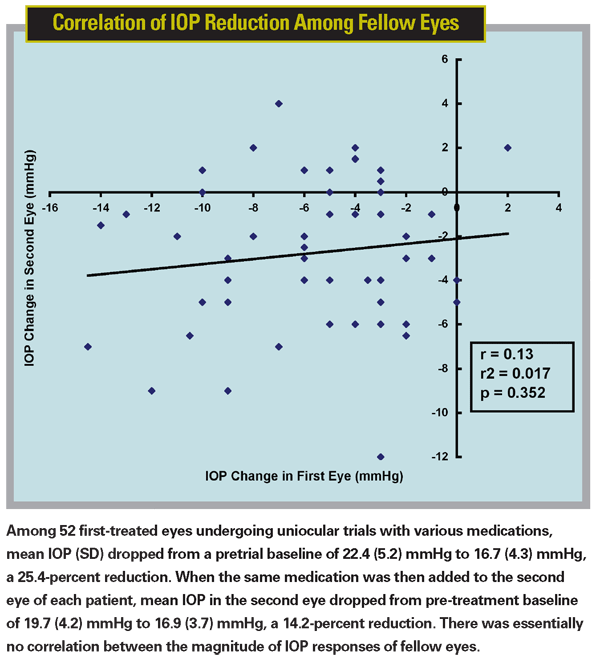
Another group found poor correlation of fellow-eye responsiveness to bilateral treatment with latanoprost; r2 ranged from 0.274 for daytime IOP measurements to 0.413 for 24-hour diurnal IOP measurements. (Young A, et al. IOVS 2006;47:ARVO E-Abstract 437.) And a retrospective study of 590 participants in the Ocular Hypertension Treatment Study found only a moderate correlation between the response of the first and second eyes to treatment with a topical beta-blocker (r2=0.185). (Bhorade AM et al. IOVS 2007;48: ARVO E-Abstract 5555)
The latter assumption—that the second eye is not affected by the first eye's treatment—has already been shown to be false, at least when beta-blockers are used. OHTS investigators reported a mean 1.5-mmHg reduction in the IOP of fellow eyes during monocular trials with beta-blockers.8 The potential contralateral effects of other IOP-lowering drug classes are less well-established, but there is clearly reason to suspect that the fellow eye has been affected by the first eye's treatment.
The Problem of Compliance
As we all know, and many studies have demonstrated, patients often fail to use medication as directed. The accuracy of the monocular drug trial depends on patient compliance. If a patient treats both eyes, or treats the wrong eye, the monocular trial won't provide useful data. And, of course, noncompliant patients often don't admit that they've failed to follow instructions. This introduces an unpredictable variable into the equation that puts any fellow eye comparison on potentially shaky ground.
The Single Measurement Problem
Given that IOP is not constant over the course of the day, what we're really trying to do is lower the patient's range of pressure. Unfortunately, we can't determine a change in range by taking a single measurement; doing so is like trying to intuit the plot of a movie from a single frame. And yet, this is precisely what we're doing when we base a clinical decision about whether a treatment is working on a single measurement.
Ironically, the Academy's Preferred Practice Pattern for POAG clearly states that when starting therapy it's best to get a number of pretreatment baseline measurements so you understand the range of IOP the eye is experiencing before treatment. But despite this acknowledgement of the importance of determining IOP range, the PPP does not suggest that you should get more than a single on-treatment IOP measurement, and therein lies the problem. You can't draw conclusions about whether you've lowered the patient's range of fluctuation with a single measurement—yet many of us do.
Possible Consequences
Suppose the result of a monocular drug trial leads you to draw the wrong conclusion about the medicine in question. This could go two ways, of course. A drop in pressure that appears to be caused by the drug but is really just an asymmetric fluctuation within the patient's current pressure range could lead you to keep the patient on an ineffective drug for several more months. In this case, you'd eventually take enough measurements to realize that the drug wasn't working, but the patient might be using an ineffective treatment for six, nine or 12 months—depending on how often you have patients back for follow-up evaluations.
Conversely, suppose the first drug you try is effective at lowering the patient's pressure range, but fluctuating pressure produces a high-end reading that still falls within the pretreatment pressure range. Most ophthalmologists would conclude that the treatment wasn't working and would switch the patient to a different option. This possibility concerns me because almost all of us now begin treatment by prescribing a prostaglandin. If you switch the patient to a different class of drugs on the basis of this one reading, you may be needlessly depriving the patient of the safest and generally most efficacious class of drugs out there.
Capturing a Range
So what is the alternative to the monocular drug trial? The obvious answer is to take multiple measurements—whether you start by treating one or both eyes. This will allow you to get an idea of the mean IOP, peak IOP and range of IOP before you make your decision about the effectiveness of a treatment—just as you did before treatment. (Obviously, if the patient is end-stage and likely to go blind in the next few weeks or months, you don't want to take extra time to make a decision. Luckily, few patients fall into this category.)
Unfortunately, there's no data to support a particular algorithm for how the post-treatment testing should be done. We don't know how many visits might be required to really determine the new pressure range. On the other hand, we should at least aim for the same number of measurements we took to determine the pre-treatment range. We do face practical limitations; for now, at least, we still have to numb the eye and have a trained observer determine the pressure. Other medical conditions don't have this problem; if a patient has diabetes, she can do home glucose monitoring. We generally only get to check our patient's pressures three or four times a year.
My current approach is that if I think a patient needs treatment, depending on how high the pressure is and how advanced the glaucoma is, I aim for two, preferably three pre-treatment pressure measurements over the course of a couple of weeks or months. Once we start treatment I continue the medication past the first visit, even if it appears that the IOP hasn't gone down (as long as the IOP is not dangerously high). Depending on the severity of the glaucoma, I may have the patient come back in a couple of weeks or in a month or two to check the pressure again. My goal is to give the newly initiated medication more than a single opportunity to show me whether or not it's working.
Even if you decide to see the patient multiple times, both before and after treatment begins, there's another potential confounding factor: the issue of exam timing. A particular patient may always come into the office for an appointment at the same time of day. If there's a circadian rhythm that governs our IOP fluctuations and I see the patient at 8:00 a.m. every time, then I'm going to vastly underestimate her IOP variability. Or, if a patient always comes in right before lunch when his morning dose of medicine is working optimally, I'll always see the best-case pressure. For this reason, it makes sense to try to see the patient at different times of day, both pre- and post-treatment, to get a better sense of the patient's pressure fluctuation range.
Many doctors advocate the opposite: Check the patient's pressure at the same time at every visit to eliminate the diurnal variability factor. This sounds reasonable, but I haven't seen any data that conclusively demonstrates consistency in diurnal variation. In fact, some data suggests that diurnal curves are not reproducible from day to day.1,2 (Realini, AD et al. IOVS 2006;47: ARVO E-Abstract 4464) A host of factors might cause it to shift—how well and long the patient slept, a change in activity from day to day, and so forth. So, measuring at the same time every day may not guarantee that circadian shifts have been factored out of the equation.
The Bottom Line
All of this brings us back to the central issue: We're trying to alter, and monitor, a range of pressure fluctuation. Basing our judgment about the success of treatment on a single post-treatment measurement and a comparison between eyes is fraught with peril. Although an error resulting from this may not cause grave damage to the patient's vision, it could lead to less than ideal treatment.
In short, I believe there is sufficient reason to reconsider the wisdom of drawing conclusions about a treatment's effectiveness on the basis of a monocular drug trial. It's an approach that saves time and effort, but the evidence suggests that it may be shortchanging some patients.
1. Katavisto M. The diurnal variations of ocular tension in glaucoma. Acta Ophthalmol (Copenh) 1964;78(suppl):1-131.
2. Wilensky JT, Gieser DK, Dietsche ML,
3. Sit AJ, Liu JH, Weinreb RN. Asymmetry of right versus left intraocular pressures over 24 hours in glaucoma patients. Ophthalmology. 2006;113;3:425-30.
4. Liu JH, Sit AJ, Weinreb RN. Variation of 24-hour intraocular pressure in healthy individuals: right eye versus left eye. Ophthalmology. 2005;112;10:1670-5.
5. Realini T, Barber L, Burton D. Frequency of asymmetric intraocular pressure fluctuations among patients with and without glaucoma. Ophthalmology. 2002;09;7:1367-71.
6. Realini T, Vickers WR. Symmetry of fellow-eye intraocular pressure responses to topical glaucoma medications. Ophthalmology 2005;112:599-602.
7. Realini T,
8. Piltz J, Gross R, Shin DH et al. Contralateral effect of topical beta-adrenergic antagonists in initial one-eyed trials in the ocular hypertension treatment study. Am J Ophthalmol 2000;130:441-453.











