|
THE LATEST RESEARCH
|
|
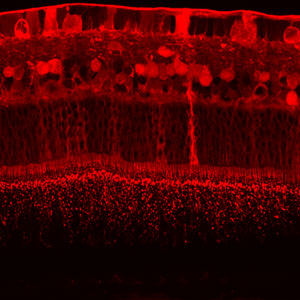
| The retina of an albino rabbit 24 hours after
a 2.5-mg intravitreal injection of bevacizumab (Avastin) as part
of a 10-rabbit study (Shahar J, et al.). The vertical streak is
a Muller cell. Staining in the rod outer segments is visible at
the bottom of the image. Confocal immunohistochemistry showed full-thickness
penetration at 24 hours, which was absent at four weeks. (Image
courtesy of Robert L. Avery, MD.) |
Effects of Intravitreal Bevacizumab
Five recently published studies evaluated intravitreal bevacizumab (Avastin)
as treatment for several conditions as well as its effects on human
macular function and its level of retinal toxicity and penetration in
rabbits.
In a short-term retrospective study (Iturralde D, et al.) of 16 eyes
(15 consecutive patients) with macular edema secondary to central retinal
vein occlusion, treatment with at least one intravitreal injection (1.25
mg/0.05 mL) of bevacizumab decreased the edema and improved visual acuity.
The mean number of injections was 2.8 per eye. Mean central macular
thickness was 887 µm at baseline and 372 µm at one month
(p<0.001). Mean visual acuity improved from 20/600 at baseline to
20/138 at the last follow-up visit, which was a mean of three months
after the first injection (p<0.001). In 14 of the 16 eyes, visual
acuity improved, as defined by a halving of the visual angle. No adverse
events, including endophthalmitis, clinically evident inflammation,
increased intraocular pressure, retinal tears, retinal detachment, or
thromboembolic events, occurred.
Nine of the patients in the study had previously received intravitreal
injections of triamcinolone, which resulted in no improvement or excessive
intraocular pressure.
In another study (Spaide RF, et al.), involving two patients, intravitreal
injection of bevacizumab (1.25 mg/0.05 mL) led to regression of neovascularization
and resolution of vitreous hemorrhage associated with proliferative
diabetic retinopathy. In both patients, the vitreous hemorrhage was
extensive enough to preclude panretinal photocoagulation. In both patients,
retinal neovacularization regressed at one month, and the vitreous hemorrhage
partially resolved at one week and was nearly completely resolved at
one month. No adverse events occurred. Improvement in visual acuity
was noted within the first week after injection. At one month, one patient
improved by two lines, and the other improved by five lines. One patient
received a second injection at the one-month follow-up (slight leakage
from neovascularization on the nerve), and one patient received a second
injection at three months (early signs of reperfusion of retinal neovascularization).
In a third study (Maturi RK, et al.), nine patients treated with intravitreal
bevacizumab for neovascular age-related macular degeneration (AMD) underwent
multifocal electroretinography (mf-ERG) or Ganzfeld electroretinography
(G-ERG) before treatment. The five G-ERG patients were tested at one
week after injection. The four mf-ERG patients and four of the five
G-ERG patients were tested at one month after injection. All mf-ERG
tests showed improvement in macular response at one month. The average
improvement in the response density of the central 15 degrees was 35
percent (range 11-65 percent). G-ERG testing showed no significant changes
in response, although some variation in amplitude and implicit time
was observed at different testing times. Visual acuity improved in the
majority of patients, and central subfield thickness as measured by
optical coherence tomography decreased from 298 µm to 274 µm.
A fourth study (Manzano RP, et al.), involving 12 New Zealand albino
rabbits, tested four concentrations of bevacizumab (500 µg/0.1
mL, 1.0 mg/0.1 mL, 2.5 mg/0.1 mL, and 5.0 mg/0.2 mL). Each concentration
was injected intravitreally in one eye of each of three rabbits, and
the contralateral eye was injected with 0.1 mL of balanced saline solution.
The rabbits were observed for 2 weeks. The rabbits were tested with
ERGs at baseline and day 14. Histologic and ERG testing indicated no
retinal toxicity, but some inflammatory cells were detected in the vitreous
at the 5-mg dose.
In a fifth study (Shahar J, et al.), one eye of 10 albino rabbits was
injected intravitreally with 2.5 mg/0.1 mL of bevacizumab and the other
eye was injected with 0.1 mL of saline. Bevacizumab was found to be
nontoxic. The researchers performed ERG testing at three hours, three
days, and one, two and four weeks after injection. They recorded visually
evoked potential (VEP) at four weeks. They used confocal immunohistochemistry
to determine penetration into the retina. ERG responses in the control
and experimental eyes were similar throughout follow-up. Flash VEP responses
in the experimental eyes showed normal pattern and amplitude and were
not different than those recorded by stimulation of the control eye
alone. Full thickness retinal penetration was present at 24 hours and
absent at four weeks.
Sources: Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal
bevacizumab (Avastin) treatment of macular edema in central retinal
vein occlusion: a short-term study. Retina 2006;26:279-284. Spaide RF,
Fisher YF. Intravitreal bevacizumab (Avastin) treatment of proliferative
diabetic reinopathy complicated by vitreous hemorrhage. Retina 2006;26:275-278.
Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal
bevacizumab (Avastin) treatment. Retina 2006;26(3):270-274. Manzano
RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of
bevacizumab (Avastin). Retina 2006;26(3):257-261. Shahar J, Avery RL,
Heilweil G, et al. Electrophysiologic and retinal penetration studies
following intravitreal injection of bevacizumab (Avastin). Retina 2006;26(3):262-269.
Fluocinolone Implant for Uveitis and DME: Three-Year Results
Preliminary three-year results from the clinical trial of the fluocinolone-containing
intravitreal implant (Retisert) for chronic non-infectious posterior
segment uveitis showed that inflammation was controlled in significantly
more implanted eyes than non-implanted eyes, but less controlled than
was reported at two years. The findings suggested that some eyes might
require replacement of the implant between the two-year and three-year
time periods, which is consistent with the implant’s FDA approval
for a treatment period of 30 months.
The 278 patients in the multicenter, randomized, dose-masked trial were
randomized to receive either a 0.59-mg or 2.1-mg implant in one eye.
The data released represent the aggregate of the two doses. The uveitis
recurrence rate in non-implanted fellow eyes at three years was 57 percent;
the recurrence rate in implanted eyes at three years was 33 percent
(p<0.001). The recurrence rate at two years in implanted eyes was
11 percent.
At three years, baseline visual acuity improved by three lines or more
in 22 percent of implanted eyes vs. 6 percent of non-implanted fellow
eyes. Two percent of implanted eyes required removal of the implant
to control intraocular pressure; 92 percent required cataract extraction;
and 45 percent required filtration surgery.
Preliminary three-year results from the clinical trial of the fluocinolone
implant for diabetic macular edema showed that the implant resolved
edema at the center of the macula and improved visual acuity by three
or more lines in a significant proportion of eyes.
In the multicenter, randomized, controlled trial, 197 patients were
randomized 2:1 to receive either a 0.59-mg implant or standard of care
defined as repeat laser or observation. At three years, 58 percent of
implanted eyes exhibited no evidence of edema vs. 30 percent of eyes
receiving standard care (p<0.001). A greater than one step improvement
in diabetic retinopathy score was observed in 13 percent of implanted
eyes and 4 percent of standard-of-care eyes (p<0.001).
Twenty-eight percent of implanted eyes gained three or more lines of
visual acuity compared with 15 percent of standard-of-care eyes (p<0.05).
Among (phakic) implanted eyes, 95 percent required cataract extraction.
Twenty-eight percent of implanted eyes required a filtering procedure,
and 5 percent required explantation to control intraocular pressure.
More complete results from each study will be presented in May at the
annual meeting of the Association for Research in Vision and Ophthalmology.
Source: pSivida Limited, February 2006.
Mouse Study Adds to Understanding of Smoking and AMD
According to the results of a study in mice, cigarette smoke-related
oxidants, specifically hydroquinone, may stimulate injury to the choriocapillaris
and retinal pigment epithelium (RPE), contributing to the association
between smoking and AMD. In the study, exposure to cigarette smoke or
hydroquinone resulted in the formation of sub-RPE deposits, thickening
of Bruch’s membrane, and accumulation of deposits within Bruch’s
membrane.
The mice were fed a high-fat diet for 4.5 months and divided into two
groups. The first group was exposed to blue-green light (positive control)
or whole cigarette smoke. The second group received a purified diet
with hydroquinone (0.8 percent) with low or high fat content for 4.5
months. A third group with no intervention served as a negative control.
Most mice fed a high-fat diet without other oxidant exposure exhibited
normal morphology, but those exposed to whole cigarette smoke or hydroquinone
exhibited a variable degree of basal laminar deposits and diffusely
thickened Bruch’s membrane.
The researchers reported that understanding the molecular mechanisms
that cause these changes could lead to protective therapies for smokers
and non-smokers. They also pointed out that hydroquinone is a component
of air pollution, and the incidence of AMD is increasing in areas with
high pollution rates.
Source: Espinosa-Heidmann DG, Suner IJ, Catanuto P, et al.
Cigarette smoke-related oxidants and the development of sub-RPE deposits
in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci
2006;47:729-737.
Final Results from Drusen Laser Study Published
As indicated by previously published interim results from the Drusen
Laser Study, published final results do not support prophylactic laser
treatment for the fellow eye in patients with neovascular age-related
maculopathy (ARM). Based on the final results, the researchers who conducted
the study also concluded that the role of prophylactic laser in patients
with bilateral drusen remains unclear.
In the prospective, interventional, randomized, controlled clinical
trial conducted at five hospital centers, patients with neovascular
ARM and drusen in the study eye (unilateral group) were randomized to
laser treatment or no laser treatment. The eyes of patients with bilateral
drusen were randomized to right eye, laser or no laser, and left eye,
alternative. Laser treatments comprised 12 argon spots. Best-corrected
visual acuity and signs of CNV were monitored for three years.
In the unilateral group, 21 of 73 patients (28.8 percent) treated with
laser lost vision compared with 13 of 66 patients (19.7 percent) not
treated with laser (p=.214). Among the 91 patients treated with laser,
choroidal neovascularization (CNV) incidence was 29.7 percent compared
with 17.65 percent of the 85 patients not treated with laser (p=.061).
Onset of CNV occurred approximately six months earlier in the patients
who were treated with laser (p=.05).
In the bilateral group, six of 72 laser-treated eyes (8.3 percent) lost
vision compared with 10 of 72 (13.9 percent) fellow eyes (p=.3877).
The incidence of CNV was 11.6 percent among 103 eyes treated with laser
compared with 6.8 percent among fellow eyes. No difference in onset
of CNV was observed.
Source: Owens SL, Bunce C, Brannon AJ, et al. Prophylactic
laser treatment hastens choroidal neovascularization in unilateral age-related
maculopathy: final results of the drusen laser study. Am J Ophthalmol
2006;141:276-281.
|