Anti-VEGF therapy has become the mainstay for managing patients with wet macular degeneration. While the response rate in published studies ranges from 70 to 95 percent depending on the agent used and the treatment paradigm employed, it is clearly not 100 percent, and some patients do fail treatment. It is these treatment failures that usually represent the most challenging cases. Here, I review the limited data available that relates to the management of such treatment failures and present our evolving approach for these uncommon and complicated cases.
Recognizing Treatment Failures
There is no universally agreed upon definition of treatment failure. Some clinicians identify a treatment failure when multiple anti-VEGF treatments have been applied and fluid or leakage persists on optical coherence tomography or fluorescein angiography, respectively. Others deem treatment a failure when vision loss continues despite a course of treatment. There is also the concept of "treatment disappointments," for example, when continuous monthly injections are required to keep the macula dry or where treatment produces a decrease in edema but no improvement in vision.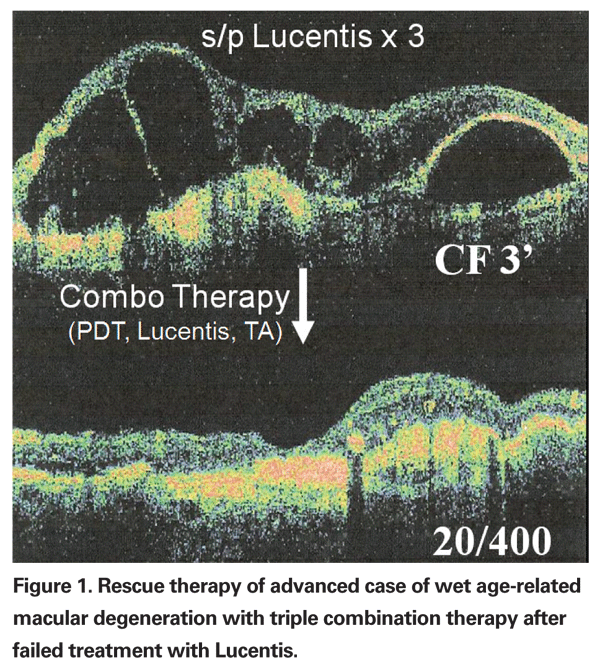
For practical purposes, I define treatment failures as those patients who no longer respond with an improvement in macular edema on OCT or a reduction of leakage on fluorescein angiography following treatment with an anti-VEGF agent. Figure 1 illustrates a treatment failure of Lucentis in an advanced case of wet age-related macular degeneration with subsequent response to combination therapy utilizing Lucentis, Visudyne and intravitreal injection of unpreserved triamcinolone acetate.
Macugen Treatment Failures
The approach to treatment failures clearly relates to the agent associated with the treatment failure. In the case of pegaptanib (Macugen), for example, the approach has been straightforward: switch to an alternative anti-VEGF agent with a response rate clearly greater than that of pegaptanib. Pegaptanib was the first approved anti-VEGF treatment for the management of wet macular degeneration. This specific and selective agent is an oligonucleic aptamer that binds specifically to the subtype of VEGF most frequently associated with neovascularization—the VEGF 165 isoform. While data supports a treatment effect for Macugen with a maximal response rate of about 70 percent, there were significant treatment failures with this agent, as well as "treatment disappointments" in a substantial proportion of "responders" who continued to lose vision over time.
In contrast, the second approved anti-VEGF agent, Lucentis, exhibited a response rate of 95 percent. Thus, most retina specialists have utilized the more potent anti-VEGF agent Lucentis, and its off-label cousin, Avastin, for the management of Macugen treatment failures. As a result, Macugen is now rarely used as a sole treatment for wet AMD. In a limited study that was prematurely halted, the addition of PDT with intravitreal Macugen did not appear to improve response rates and PDT alone is not routinely used for rescue therapy.
Lucentis, Avastin Treatment Failures
Lucentis and Avastin are anti-VEGF agents derived from monoclonal antibodies that can bind all subtypes of the VEGF molecule. Lack of selectivity for VEGF has not been associated with feared complications such as detectable loss of normal retinal capillaries, and the much higher response compared to Macugen suggests that more VEGF isoforms may be involved in pathologic choroidal neovascularization. With a response rate of 95 percent for Lucentis and an empirically comparable rate for Avastin, failures are uncommon. Failures are difficult to predict but one study found that the efficacy of anti-VEGF therapy appears to depend on the initial lesion size and initial reading ability. With a bigger choroidal neovascular size and a lower reading ability, the chance of responding appears to be reduced.
Treatment protocol may also affect response rates and efficacy. The initial Lucentis studies—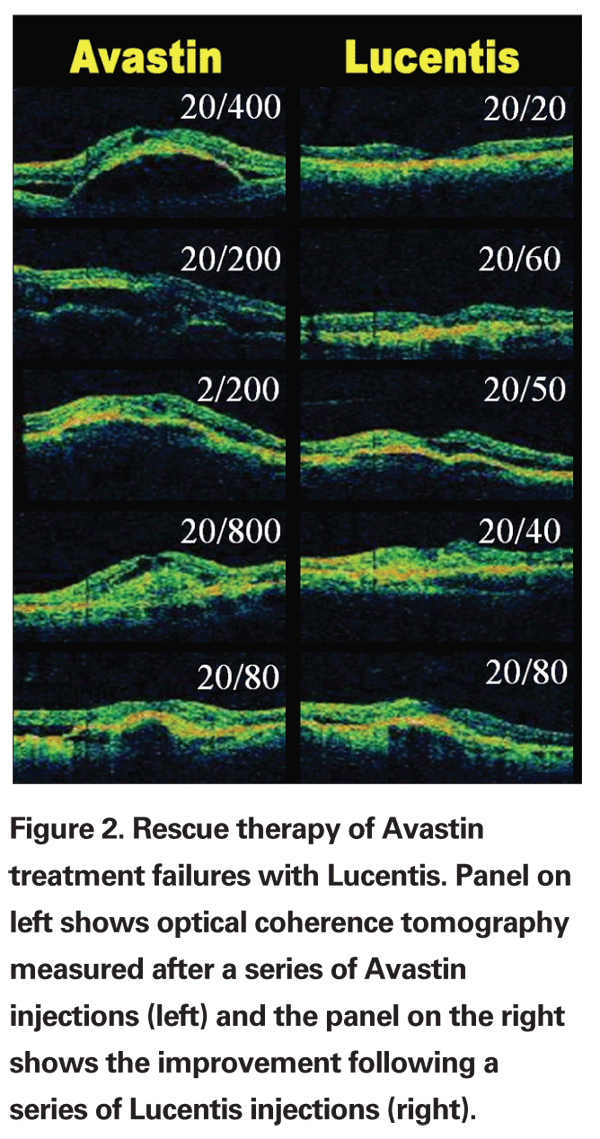
In the PrONTO study, a treat-and-observe protocol was utilized in which patients received three initial monthly injections of Lucentis and were retreated only when: a) they lost more than five letters of vision and had any fluid on OCT; b) they developed new retinal hemorrhage or classic choroidal neovascularization; or c) there was an increase of >100 µm of edema documented on the OCT.
While the visual acuity results achieved at one year in the PrONTO study were comparable to the results achieved with monthly dosing in the Phase III MARINA and ANCHOR trials, the percentage of patients with an overall decrease in vision was higher, raising concerns about the treat-and-observe approach utilized in the study.
Today, many clinicians utilize a "treat-and-extend" protocol where a series of two or three injections is followed by extending the follow-up and retreatment visits to the period of time where fluid recurs. In some patients the retreatments can be extended from every four weeks to every six weeks and so on until reinjections are scheduled three months apart. If edema recurs between visits the protocol is reinitiated. This protocol may reduce the rate of treatment failures for both Avastin and Lucentis.
When the SAILOR study allowed treatment of patients with wet AMD after they had failed other therapies, we gained experience with the use of Lucentis as rescue therapy. In my practice, the majority of patients were receiving Avastin off-label, since published data suggested this was the most effective therapy for treatment of non-study patients with wet AMD. Of the 13 Avastin treatment failures I treated with Lucentis, 10 responded with a reduction in edema and/or improvement in best corrected visual acuity.
Examples of six best responses on OCT (greatest reductions in edema) are shown in Figure 2. All 13 patients had occult choroidal neovascularization with a fibrovascular pigment epithelial detachment. One patient developed a retinal pigment epithelial tear outside of the fovea but still recovered 20/25 vision for nearly one year until he recurred and was stabilized with a triple-therapy regimen (Avastin/Visudyne/Dexamethasone, see below).
Combination Therapies
Combination therapy is most commonly used as rescue therapy for patients failing anti-VEGF treatment. The treatment protocols are based on the idea that a multi-pronged approach may be more effective than any single approach since multiple factors contribute to wet AMD including: 1) abnormal VEGF production; 2) inflammatory mediators; and 3) increased vascular permeability of choroidal neovascular membranes (See Figure 3). In theory, by attacking each of these three areas—VEGF with anti-VEGF agents, inflammation with corticosteroids, and vascular permeability with photodynamic therapy—we have a better chance of arresting the pathologic process altogether, if not at least for a longer period of time.
The published data support the theory and, in general, combination therapy appears to provide a comparable visual result with a longer duration of effect when compared to anti-VEGF therapy alone.
Combination therapy is also effective for managing most anti-VEGF treatment failures. A variety of combination therapies have been utilized for anti-VEGF treatment failures and in some cases as primary therapy. Most "double therapies" combine an anti-VEGF agent with Visudyne, and there is data supporting efficacy for this approach. Most investigators are utilizing a triple-therapy regimen with anti-VEGF, photodynamic therapy and corticosteroid.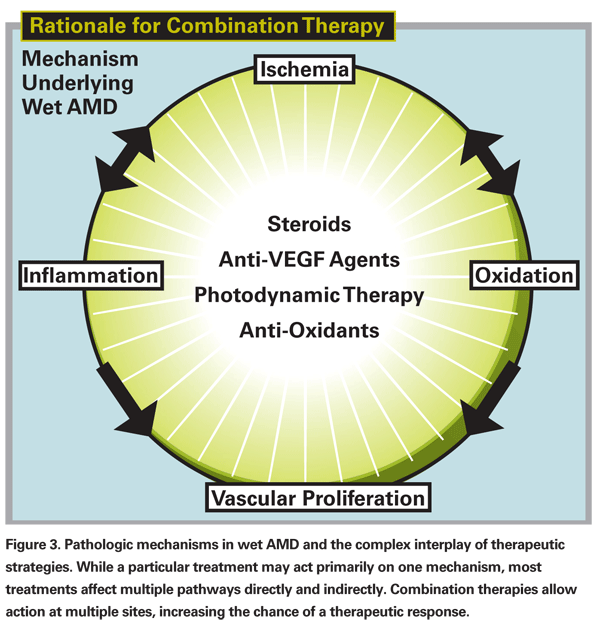
Amounts of anti-VEGF drug vary, but most commonly used are Avastin 1.25 mg/0.05 cc or Lucentis 0.3 mg/ 0.05 cc. Dexamethasone is the most commonly used corticosteroid at a dose of 6 mg/0.05 cc. These volumes allow completion of the procedure without a concomitant limited vitrectomy and appear to be effective.
While not approved for reimbursement by the Centers for Medicare & Medicare Services, many use a protocol of reduced fluence by lowering the power density by 50 percent or reducing the duration of treatment. Since combination therapy adds the use of Visudyne to the treatment protocol, the reported 1 to 4 percent risk of acute vision loss with photodynamic therapy must be disclosed to the patient.
For most retinal specialists, it is this risk that limits the use of combination therapy for primary treatment, particularly in those with relatively preserved visual acuity (>20/200) and a large fibrovascular PED or RAP (retinal angiomatous proliferation) lesions (since an RPE tear may be more likely to occur in these patients). Examples of resistant cases responding to combination therapy are shown in Figure 4.
Role of Corticosteroids
As noted above, dexamethasone is the most commonly used corticosteroid in combination "triple therapy." Unpreserved triamcinolone acetate may still be useful in select cases. I consider an intravireal triamcinolone acetate injection as an alternate to dexamethasone for triple therapy in patients who: 1) have a history of improvement with IVTA in the past; 2) are not known corticosteroid responders; 3) are pseudophakic; or 4) have evidence for vitreous inflammation on clinical examination. While wet AMD is likely the result of multiple factors, as shown in Figure 4, in some patients, one factor may predominate over another, and thus longer acting corticosteroids such as TA may play a more critical role in the treatment of select patients. Some clinicians still utilize combination therapy with IVTA and Visudyne and this may still be appropriate in select cases.
In rare cases I have used a treat-and-observe protocol with IVTA alone for managing pseudophakic patients with wet AMD. A new formulation of IVTA has recently been released by Alcon, and Allergan now has a sustained-release unpreserved formulation awaiting approval. The use of the formulation in wet AMD should be explored.
Other Treatment Options
Other treatment options are still available for wet AMD, although anti-VEGF therapy has greatly curtailed their use. Transpupillary Thermotherapy (TTT) is still reimbursed by CMS for the treatment of wet AMD. The TTT4CNV clinical trial, however, did not find a significant difference between TTT and sham (47 percent vs. 43 percent, respectively). While the results of the submacular surgery trial (SST) as well as a larger meta-analysis of additional studies argue against the use of surgery in patients with wet AMD, surgical excision of CNV is still utilized in select patients, particularly those who have failed anti-VEGF therapy and have a more clearly defined subretinal (type I) rather than sub-RPE (type II) lesion. Type I lesions are typically found in non-AMD patients.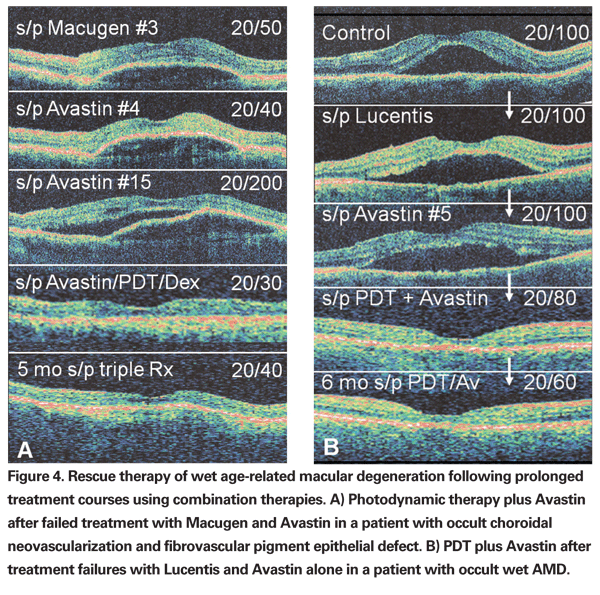
In select AMD patients surgery may be considered for treatment failures, particularly those with extensive subretinal fluid, recent but severe vision loss and a small lesion relative to the area of involvement. Submacular hemorrhages associated with wet AMD are still pneumatically displaced in the office or, in the OR with subretinal TPA in select cases. Focal radiation therapy with an intravitreal probe (Epi-Rad90 Ophthalmic System, NeoVista,
Role of NSAIDs
There is now evidence that non-steroidal anti-inflammatory drops may suppress VEGF activity and inhibit choroidal neovascularization. Additional studies are required to determine if NSAIDs are useful as adjunctive therapy in the management of choroidal neovascularization.
Prognosis of Treatment Failures
An empiric observation of treatment failures suggests a worst prognosis for visual acuity. Many of these patients have a fibrotic element associated with the choroidal neovascular membrane and have undergone unsuccessful treatment for a long period of time. In my small series of 13 patients who failed Avastin prior to the availability of Lucentis, all had 20/200 or worse visual acuity.
Diagram for Treatment Failures
Managing treatment failures requires both a creative and consistent approach and the major strategies are summarized in Figure 5. Using the most potent currently available anti-VEGF agents, stabilization of vision and edema can be achieved in about 95 percent with a treat-and-observe or treat-and-extend protocol. The minority, 5 percent, are treatment failures and continue to experience leakage, growth or progressive vision loss.
When these lesions are associated with relatively poor vision (<20/200), and there is a poor response to the initial treatment protocol, prompt treatment with combination therapy should be considered. If the vision is relatively preserved (>20/200) and the lesion is small, continued monotherapy may be reasonable, although for lifestyle reasons, combination therapy may be selected by the patient if frequent injections have become a burden. When combination therapy is successful, maintenance treatment may still be required with periodic anti-VEGF injections given as needed.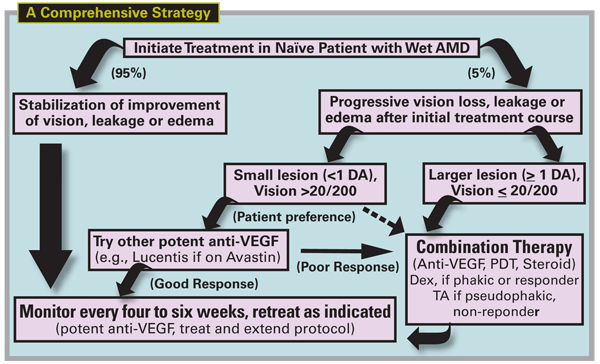
Recognizing an anti-VEGF treatment failure early on may improve outcomes. Having a clear definition of treatment failure—persistent fluid on OCT no longer responsive to anti-VEGF injections—will help you identify these patients. Following consistent "treat and observe" or "treat and extend" protocols may also reduce failure rates. Informing patients of the off-label and unproven nature of some rescue therapies is critical. When possible, enroll treatment failures in clinical trials to help us determine which treatment options may be best for these patients.
Dr. Gallemore is an assistant clinical professor of ophthalmology at Jules Stein Eye Institute, UCLA School of Medicine. He is also the director and founder of the Retina Macula Institute,
1. Smith BT, Shah GK. Combination Therapy for Age-related Macular Degeneration. Barnes Retina Institute.
2. Lux A, Llacer H, Heussen FMA, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol 2007;91:1318-1322.
3. Ng EW, Shima DT, Calias P, Cunningham ET Jr., Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 2006;5:123-32.
4. Kaiser P, et al. IOVS 2007;48:ARVO E-Abstract 2870.
5. Ongoing Treatment for Patients with Neovascular AMD. Retinal Physician, Oct. 2007.
6. Fung AE,
7. Romero RM, et al. 2007;48:ARVO E-Abstract 72.
8. Falkner CI, Leitich H, Frommlet F, Bauer P, Binder S. The end of submacular surgery for age-related macular degeneration? A meta-analysis. Graefe's Arch Clin Exp Ophthalmol 2007;245:490-501.
9. Hawkins BS, et al and Submacular Surgery Trials Research Group. Surgical removal vs. observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic: I. Ophthalmic findings from a randomized clinical trial: Submacular Surgery Trials (SST) Group H Trial: SST Report No. 9. Arch Ophthalmol 2004;122:1597-611.
10. Haupert CL, McCuen BW 2nd, Jaffe GJ, Steuer ER, Cox TA,
11. Reichel E et al. IOVS 2007;48:ARVO E-Abstract 3657.
12. Combination Therapy: Lucentis (Ranibizumab Injection) and Xibrom (Bromfenac Ophthalmic Solution) 0.09% in the Treatment of Choroidal Neovascular Membrane Secondary to Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci, ARVO Abstracts, 2008 (in press).
13. Amrite AC, Kompella UB.Celecoxib inhibits proliferation of retinal pigment epithelial and choroid-retinal endothelial cells by a cyclooxygenase-2-independent mechanism. J Pharmacol Exp Ther. 2008 Feb;324(2: 749-58. Epub 2007 Nov 21.
14. Takahashi K, Saishin Y, Saishin Y, Mori K, et al. Topical nepafenac inhibits ocular neovascularization.Invest Ophthalmol Vis Sci. 2003 Jan;44(1): 409-15.











