Although a few serious com- plications have been associated with intraocular lens materials in the past, current materials and manufacturing methods have advanced to the point that many surgeons perceive the available IOL materials as more or less interchangeable. Nevertheless, different materials do have different properties that can have a bearing on multiple concerns, including ease of implantation and the likelihood of complications such as posterior capsular opacification and endophthalmitis. (They may also have different refractive indices, which affects thickness and may increase or decrease visual disturbances after implantation.)
Here, several surgeons and researchers share some of what they've discovered about how today's IOL materials impact PCO, endophthalmitis and calcification.
The Great PCO Debate
Nick Mamalis, MD, professor of ophthalmology at the
PCO is encountered fairly often by cataract surgeons, making it a topic of widespread interest. "The one material with which PCO has been a significant issue in the past is hydrophilic acrylic, or hydrogel," says Dr. Mamalis. (He admits to having limited experience with some of the hydrophilic IOLs not currently approved here in the
Yet even among hydrophobic IOLs the incidence of PCO is not equal among different lenses. David J. Apple, MD, formerly a professor of ophthalmology and pathology and director of the David J. Apple Laboratories for Ophthalmic Devices Research at the
Dr. Apple's earlier results ignited a debate that's still ongoing: Was the Acrysof's lower rate of PCO attributable to the hydrophobic acrylic the lens optic is made of, or the lens design—in particular, the use of a square posterior edge?
Lens Material vs. Square Edge
"Manufacturers have discovered that it's very important to have a relatively sharp, square edge to the posterior IOL optic, in addition to having the lens fit well into the capsular bag," notes Dr. Mamalis. "The square edge of the optic can block the lens epithelial cells from growing across the posterior capsule. This effect has been well-demonstrated in hydrophobic acrylic, silicone and PMMA lenses. If you have a 360-degree barrier and the lens capsule is tightly adherent to the posterior part of the IOL, it will decrease the rate of PCO. So you can overcome potential material issues by using a good lens design."
Clinical research has provided support for this hypothesis. For example, Dr. Mamalis has taken part in studies at the
Dr. Mamalis says the square-edge approach has also worked with hydrophilic lenses. He was the FDA study monitor for the clinical trials of the recently approved Rayner C-flex single-piece acrylic IOL, "They've manufactured it so there is a 360-degree sharp edge all the way around the posterior part of the optic," he says. "Instead of the haptics inserting in the same plane as the optic, breaking up the 360-degree edge, the haptics insert slightly anterior to the surface. We found the incidence of YAG capsulotomy was very good three years postop—equivalent to what we see in good hydrophobic acrylic or silicone lenses."
Despite the evidence supporting the importance of a sharp optic edge, studies conducted by Liliana Werner, MD, PhD, a research associate professor at the John A. Moran Eye Center and director of preclinical research at the Berlin Eye Research Institute in Germany, have found significant differences between lens materials that likely contribute to their ability to minimize PCO. 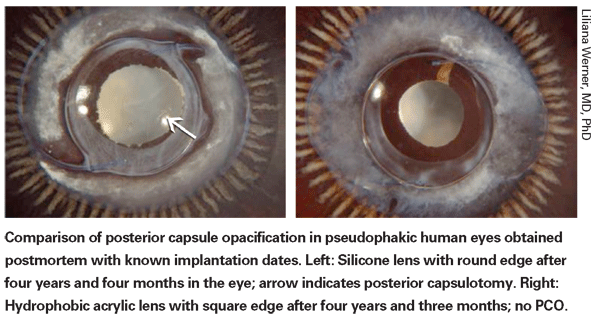
One of her studies, conducted with Reijo J. Linnola, MD, PhD, demonstrated the presence of a sandwich-like structure between capsule tissue and the IOL surface, consisting of a layer of cells—one cell thick—trapped between layers of fibronectin, in 12 of 14 autopsy eyes that had been implanted with soft acrylate IOLs.1 In comparison, this was found in only three out of 10 PMMA lenses, one out of 10 silicone lenses, and 0 out of four hydrogel lenses. The study authors noted that this appeared to be a true bioactive bond, and could be one reason PCO rates are lower when a soft acrylate is implanted. Another study found that collective protein adhesion differed significantly between soft acrylate IOLs and PMMA or silicone IOLs, with significantly more fibronectin adhering to the surface of hydrophobic soft acrylic lenses than PMMA or silicone lenses.2
In fact, the tackiness or stickiness of the lens material, which differs widely between materials, may be a significant factor in preventing PCO. (The Acrysof material is one of the tackiest.) Dr. Apple has said he believes the bioadhesive nature of the Acrysof's hydrophobic material may account for its reduced PCO by helping it to adhere to the capsule, sequestering the IOL in the bag. And apart from favoring adherence to the capsule, the tackiness of the material may directly help to inhibit PCO. A recent study conducted at Toho University in Tokyo, for example, found that among similarly shaped hydrophobic acrylic IOLs, those made of more adhesive material (as indicated by a tackiness tester) inhibited human lens epithelial cell migration and posterior capsular opacification in vitro significantly more than less-adhesive hydrophobic acrylics.3
Manfred R. Tetz, MD, director of Augentagesklinik Spreebogen
In the final analysis, Dr. Werner sees both material and design as important in terms of resisting PCO. "I do believe there are differences among materials," she says. "The studies we did with Dr. Linnola on protein adhesion comparing different IOL materials clearly demonstrated those differences. However, I believe that in terms of PCO prevention, a square edge on the posterior optic surface does the majority of the job."
The Micro-Edge Factor
To help clarify the interrelationships between PCO, lens materials and edge design, Dr. Tetz conducted a series of studies looking at edge sharpness to find out just how sharp an edge has to be to stop the spread of lens epithelial cells—and to discover how sharp the edges actually are on different manufactured lenses. The first two studies in the series were recently published.4,5
"We systematically looked at the edges of lenses using a special version of scanning electron microscopy," explains Dr. Tetz. "The first study involved PMMA lenses specially created with different kinds of sharp edges. The idea was to find out how sharp a "square" edge has to be for epithelial cells to identify the edge as a barrier beyond which they shouldn't grow, in a controlled in-vitro setting.
"First, we measured the difference between the sharpness of an ideal rectangular edge and the sharpness or roundness of the different real lens edges using image analysis software on a micrometer scale," he continues. "Then, we put the lenses in a cell culture model and watched to see which edges the cells would grow past.
"In the second study, we took a group of commercially available hydrophobic lenses, including acrylics and silicones, and looked at their edge designs," he says. [For an example, see further below.] "Then we correlated that with data from the literature about how individual lenses behave in a clinical setting. What we found, for example, is that some silicone lenses actually have perfectly square edge designs at a micrometer scale, like the original Pharmacia [now AMO] Tecnis lens, and some HumanOptics lenses. And looking at the literature we found that these particular lenses behave very well clinically. In fact, in some studies they were able to perform as well as or better than the Acrysof, which is considered the standard in this area." Dr. Tetz notes that his group found slightly different edges on different Acrysof designs, but all were within the range that should block epithelial cell migration. "Ironically," he notes, "some companies' lenses labeled 'square edge' have edges that are much too rounded to stop cell migration."
Dr. Tetz says the third paper in the series will discuss hydrophilic lenses (mostly available outside the
Ultimately, Dr. Tetz says that this series of studies supports the theory that the most influential factor in prevention of PCO is the edge design. "I'm not saying that the material plays no role," Dr. Tetz notes. "Hydrophilicity and hydrophobicity influence the fibrosis of the anterior capsule; we see more with some silicones and less with some hydrophilic materials. [For example, see reference 6.] The material also influences the speed at which both anterior and posterior capsule leaflets seal, as demonstrated by Okihiro Nishi.7 If the leaflets seal quickly because of the material, as they appear to do with the Acrysof and PhacoFlex II lenses, that's likely to contribute to preventing PCO."
The material also may affect the development of PCO by constraining the way the lens is manufactured, thereby possibly limiting the sharpness of a "square" edge. "Most hydrophilic lenses are lathe-cut or drilled in a dry state," observes Dr. Tetz. "This allows you to make very sharp edges. But the moment you put them in water, the material swells and increases in volume by 15 or 20 percent. The micro-edge gets rounder and smoother as it swells. 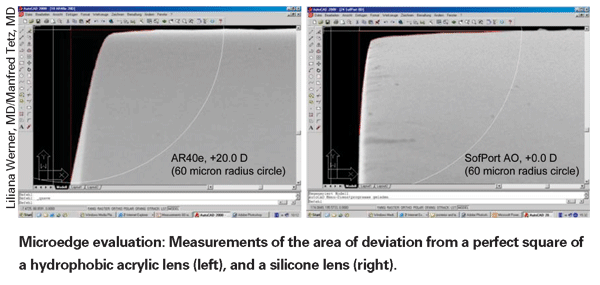
"On the other hand, most silicone lenses are still molded," he continues. "If you're using very precise molds, you can get very perpendicular edges." (However, Dr. Tetz notes that a variety of hydrophobic lenses are still polished in tumbling machines; this makes the optics smooth and round but de-sharpens the edges.) "The bottom line is that the material and the manufacturing process can both influence the sharpness of the edge," he says. "That's another way they can contribute to the likelihood of PCO developing."
Lens Material and Endophthalmitis
In the past two years, unexpected data from the European Society of Cataract and Refractive Surgery endophthalmitis study has drawn considerable attention. The purpose of the partially masked, randomized, placebo-controlled study was to prospectively evaluate the prophylactic effect of intracameral cefuroxime injection and/or perioperative levofloxacin eyedrops on the incidence of endophthalmitis after cataract surgery in about 16,000 patients. The data showed a significant benefit associated with the injection.9 But further analysis of the data looking for other factors that might have been associated with endophthalmitis rates turned up an unexpected finding: an apparent 3.3-fold increase in the likelihood of endophthalmitis associated with the use of silicone intraocular lenses.10 Peter James Barry, MD, head of the department of ophthalmology at St. Vincent's University hospital in Dublin, and chairman of the study, offered these thoughts on the significance of this finding.
"We certainly didn't anticipate the apparent increase in endophthalmitis associated with silicone lenses," he says. "In the study, surgeons were allowed to choose the lens they implanted, so this aspect of the trial wasn't randomized. It turned out that they used approximately 75 percent acrylic and 25 percent silicone lenses, plus a small number of other types.
"When we first saw the data, we were excited but not sure what to make of it," he continues. "Did this have something to do with the silicone itself, or the fact that silicone is hydrophobic? So, after the original report, we compared the data for hydrophobic and hydrophilic lenses. We found that if you used a silicone hydrophobic, your risk of endophthalmitis in the study was about one in 300, in round numbers. If you used a hydrophilic acrylic, it was about one in 600. If you used a hydrophobic acrylic, it was about one in 900. So the trend was toward the silicone material rather than the hydrophobic nature of the material. If the problem was hydrophobicity, the hydrophobic acrylics wouldn't have had the lowest incidence of all. Again, we were surprised."
Contradictory Data
Dr. Barry notes that data from some other sources has not agreed with these findings. "There was a large prospective study done by a Japanese surgeon named Yasunori Nagaki, who randomly allocated silicone and acrylic lenses in 12,000 patients," he says.11 "He found no difference between silicone and acrylic in terms of endophthalmitis. Unfortunately, the two studies aren't really comparable because the methodologies were significantly different. In his study, patients were randomly allocated to one of three treatment groups: scleral tunnel acrylic; scleral tunnel silicone; and clear corneal acrylic. There was no clear cornea silicone allocation. In our study the vast majority of silicone lenses were implanted through clear cornea incisions. Given that difference, you can't actually say that the results of the two studies are contradictory.
"A second data source that's relevant is the latest publication of the Swedish Cataract Register," he continues. "The Swedes keep fantastic records, and they're very good about tracking endophthalmitis. (They were the ones who first used intracameral cefuroxime and showed a decrease in endophthalmitis.) If you look at their last published paper, which included data on more than 225,000 patients, their endophthalmitis rates are very similar to the rates we found in our study.12 But they did not find an increased risk of endophthalmitis associated with silicone lenses.
"In all fairness, it's possible that our results were inconclusive, given that the choice of lens in our study was not randomized," he says. "The association may reflect the sort of clustering you can get in a nonrandomized event with 16,000 patients. I think we're justified in saying that the association we found is surprising and warrants pause and reflection, but to be fair to silicone lens manufacturers, I don't think we'd be justified in claiming that our result was absolute. Switching lenses bcause of our study would probably be an overreaction."
Still the Surgeon's Choice
"Nowadays, lens material differences are very small," notes Dr. Mamalis. "Of course, there are certain circumstances when you may want to choose one material over another. If you have a patient with known retinal disease who may someday need to undergo vitrectomy surgery involving insertion of silicone oil, which will bond to the surface of a silicone IOL, it might be better to use a hydrophobic or hydrophilic acrylic lens. But this is a small number of patients.
"There are very good silicone lenses, hydrophobic acrylic and hydrophilic acrylic lenses," he concludes. "I think clinicians should use whatever lens material they feel comfortable with."
1. Linnola RJ, Werner L,
2. Linnola RJ, Werner L,
3. Katayama Y, Kobayakawa S, Yanagawa H, Tochikubo T. The relationship between the adhesion characteristics of acrylic intraocular lens materials and posterior capsule opacification. Ophthalmic Res 2007;39:5:276-81.
4. Tetz M, Wildeck A. Evaluating and defining the sharpness of intraocular lenses: part 1: Influence of optic design on the growth of the lens epithelial cells in vitro. J Cataract Refract Surg. 2005;31:11:2172-9.
5. Werner L, Müller M, Tetz M. Evaluating and defining the sharpness of intraocular lenses: microedge structure of commercially available square-edged hydrophobic lenses. J Cataract Refract Surg. 2008;34:2:310-7.
6. Vock L, Georgopoulos M, Neumayer T, Buehl W, Findl O. Effect of the hydrophilicity of acrylic intraocular lens material and haptic angulation on anterior capsule opacification. Br J Ophthalmol. 2007;91:4:476-80.
7. Nishi O, Nishi K, Akura J. Speed of capsular bend formation at the optic edge of acrylic, silicone, and poly(methyl methacrylate) lenses. J Cataract Refract Surg 2002;28:3:431-7.
8. Leydolt C, et al. Long-term effect of 1-piece and 3-piece hydrophobic acrylic intraocular lens on posterior capsule opacification: a randomized trial. Ophthalmology 2007;114:1663-1669
9. Barry P, Seal DV, Gettinby G, Lees F, Peterson M, Revie CW; ESCRS Endophthalmitis Study Group. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Preliminary report of principal results from a European multicenter study. J Cataract Refract Surg. 2006;32:3:407-10
10. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons (ESCRS). Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 2007;33:6:978-8
11. Nagaki Y, Hayasaka S, et al. Bacterial endophthalmitis after small-incision cataract surgery. effect of incision placement and intraocular lens type. J Cataract Refract Surg. 2003;29:1:20-6
12. Lundström M, Wejde G, Stenevi U, Thorburn W, Montan P. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology. 2007;114:5:866-70.
13. Werner L, Hunter B, Stevens S, Chew JJL, Mamalis N. Role of silicon contamination on calcification of hydrophilic acrylic intraocular lenses. Am J Ophthalmol 2006; 141:35-43.
14. Werner L, Kollarits CR, Mamalis N, Olson RJ. Surface calcification of a three-piece silicone intraocular lens in a patient with asteroid hyalosis: A clinicopathologic case report. Ophthalmology 2005; 112:447-452











