Just a few years ago, most of the retina research at ARVO involved surgical options and lasers. Now, however, there's been a virtual explosion of studies of pharmacotherapeutics for everything from age-related macular degeneration to diabetic macular edema. Here's the latest on the retinal trials and studies coming to light at this year's ARVO meting.
Combination Therapy for AMD
Researchers from Sulzfeld and
Their prospective, interventional case series included 104 patients. The physicians administered a reduced-light PDT dose (42 J/cm^2 with a delivery time of 70 seconds). About 16 hours after the PDT, they administered 800 µg dexamethasone and 1.5 mg bevacizumab intravitreally. Patients were seen every six weeks and underwent fluorescein angiography every three months, or earlier if optical coherence tomography showed significant edema.
All 104 patients received one cycle of triple therapy, and five received a second triple treatment due to remaining choroidal neovascularization activity. Twenty-three patients (22 percent) received an additional injection of bevacizumab. The mean follow-up was 62 weeks. The mean increase in visual acuity was 1.78 lines (p<0.01), and the mean decrease in retinal thickness was 171 µm (p<0.01). There have been no serious adverse events.1167
Researchers from
In the prospective, randomized, controlled trial, the physicians randomized 28 patients with neovascular AMD to one of the two treatment groups. Patients in the bevacizumab group received three initial injections of the drug at a dose of 1 mg/0.04 ml at monthly intervals. Retreatment was based on OCT findings. Patients in the PDT/triamcinolone group received one PDT treatment at baseline combined with an intravitreal injection of 4 mg/0.1 ml triamcinolone on the same day. Retreatment was based on evidence of leakage on fluorescein at three-month intervals.
After a year, the average acuity had improved from 50 to 58 letters (p<0.01) in the bevacizumab group, but decreased from 46 to 43 letters (p=0.4) in the PDT/triamcinolone group (p=0.02 between both groups). The mean central retinal thickness decreased from 357 µm at baseline to 244 µm (p<0.01) in the bevacizumab group and from 326 µm to 254 µm (p=0.1) in the combination group (p=0.8 between both groups). After a year, the bevacizumab patients had received an average of 6.8 out of 13 possible injections, while the combo patients had received 1.9 out of five possible treatments. There were no drug-related adverse events during the year of follow-up.1168
Researchers from
In the study, patients were grouped on the basis of their CNV patterns on fluorescein angiography. Eyes in group 1 (n=52) had predominantly classic lesions while eyes in group 2 (n=106) had mainly occult lesions. Patients in both groups were treated with a limited core pars plana vitrectomy, intravitreal steroids and intravitreal bevacizumab. The patients in group 1 were also pretreated with PDT. At 14 months post-treatment, group 1's acuity increased by 1.6 lines (p<0.01), while group 2's increased by 1.2 lines (p<0.01). Macular thickness decreased by 211 µm (p<0.01) in group 1 and by 195 µm in group 2 (p<0.01). Adverse events included an IOP rise in 11 eyes (10 percent), pseudohypopyon in two eyes (1.9 percent), and a rhegmatogenous retinal detachment in one eye (0.9 percent). The retreatment rate was 25 percent in group 1 and 55 percent in group 2. The researchers say that they believe the induction of a posterior vitreous detachment alters the physiology of the eye in a way that complements concurrent medical therapy for wet AMD.566
Researchers from
In this one-year, Phase-III, randomized, double-masked study, 40 patients received three monthly intravitreal ranibizumab (0.3 mg) injections combined with either PDT (n=19) or sham PDT (n=21) at baseline. The researchers then repeated the ranibizumab injections if they detected disease progression based on a loss of greater than five ETDRS letters or increase of central retinal thickness by more than 100 µm on OCT.
At baseline, mean best-corrected acuity was 52.1 letters in both groups. At six months, acuity increased significantly by 11.8 letters in the monotherapy and by 7.8 letters in the combo group. The inter-group differences weren't statistically significant. At baseline, the mean proportion of leakage area/total area was 59 percent in the monotherapy group and 60 percent for the combo group. At six months, leakage percentage decreased to 10 percent in the monotherapy group and 6 percent in the combination group (the difference wasn't statistically significant). Fifty-six percent of monotherapy patients and 82 percent of combo patients didn't shown any angiographic activity at six months. The average CRT at baseline was 324 µm in the monotherapy group and 293 µm in the combo group, and decreased significantly to 231 µm and 213 µm, respectively (the difference between groups wasn't statistically significant).1170
Monotherapy for AMD
Researchers from the SUSTAIN trial, sponsored by Novartis, will share interim results of ranibizumab for subfoveal CNV due to AMD.
The open-label study recruited patients who were either new to ranibizumab or who had completed treatment with ranibizumab or PDT in the ANCHOR trial. A total of 531 patients were enrolled, but 69 ranibizumab-naïve patients were included in this interim analysis of the 12-month results. Patients received three consecutive monthly injections of ranibizumab 0.3 mg (0.5 mg for the ANCHOR patients) in a loading phase, followed by monthly visits. The researchers administered more treatment if acuity decreased by more than five letters or CRT increased by more than 100 µm. They withheld treatment if acuity was greater than 79 letters or CRT was less than 225 µm.
In the 69 patients available at a year, average acuity increased by about seven letters and the mean CRT decreased by 74.4 µm. The average number of injections administered to these 69 patients was 5.3. Fifty-two percent experienced adverse events, but only 3 percent had serious events (one retinal hemorrhage and one retinal pigment epithelial detachment).273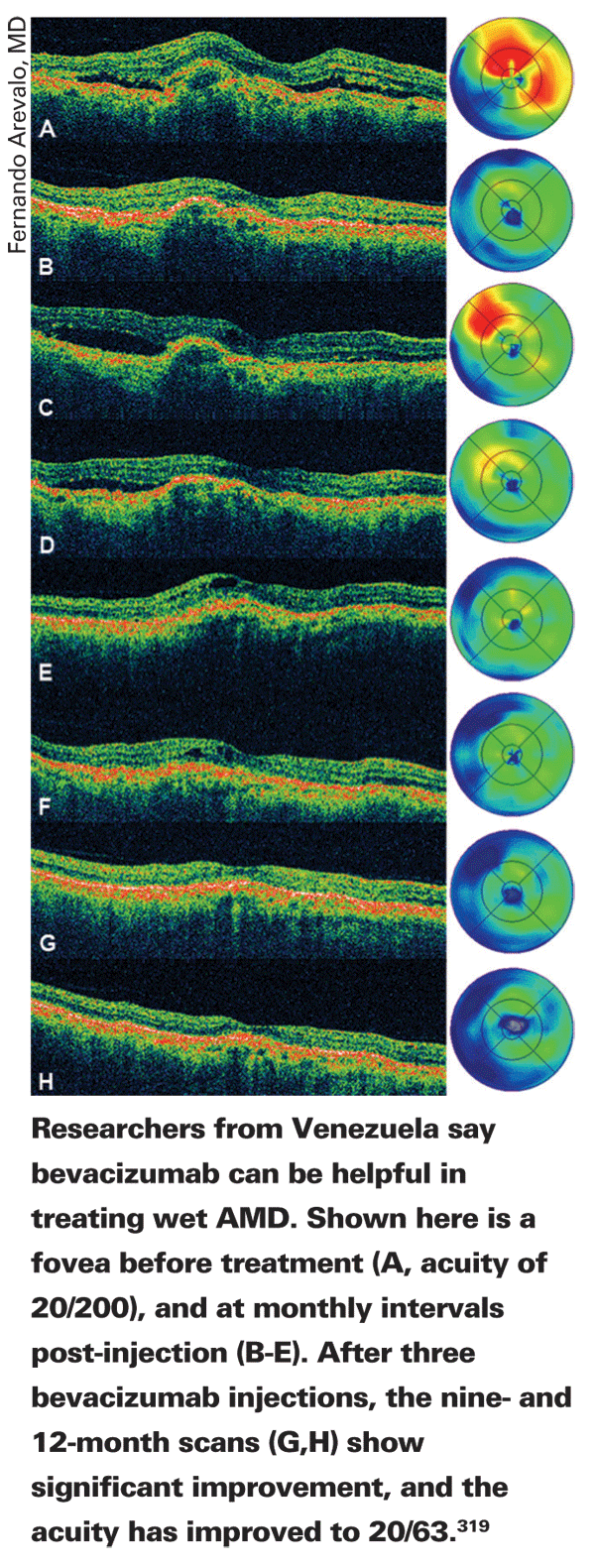
Surgeons from seven countries taking part in the Pan-American Collaborative Retina Study Group report good 12-month results with intravitreal bevacizumab for subfoveal CNV from AMD.
In the retrospective study, 90 patients (112 eyes) were analyzed, though 63 were available for this report. Their average acuity at baseline was 20/320. They were treated with at least one intravitreal injection of 1.25 mg or 2.5 mg of bevacizumab.
At one year, the average number of injections per eye was 3.5 (range: one to eight). Their acuity improved to 20/200. Central macular thickness was reduced significantly from 389 ±150 µm to 241 ±77 µm. Ocular adverse events included transient arterial hypertension in two eyes (3 percent), transient intraocular pressure increase in two eyes (3 percent), endophthalmitis in two eyes (3 percent) and transient hypotony in one (1 percent).319
Researchers from the PIER study of ranibizumab for wet AMD say that there were smaller increases in the CNV area with the drug than with sham injections. Two of the researchers are Genentech employees and a third receives financial support from the company.
The PIER study is a Phase-IIIb trial evaluating a less-frequent ranibizumab dosing schedule than that used in the pivotal
The ranibizumab groups had significantly less mean change from baseline than the sham group in total area of CNV at month 24 (0.3 mg=0.29, 0.5 mg=0.64, sham=1.90 disc areas; p<0.003) and at month 12 (0.3 mg=0.18, 0.5 mg=0.43, sham=2.08 disc areas; p<0.002). Change from baseline in total area of leakage plus retinal pigment epithelium staining did not significantly differ among groups at month 24 (0.3 mg=-1.52, 0.5 mg=-1.22, sham=-0.78 disc areas), but did at month 12 (0.3 mg=-1.41, 0.5 mg=-1.29, sham=+1.40 disc areas) (p<0.0001, each ranibizumab group vs. sham).
For each group, the mean change from baseline in total lesion area at month 12 was smaller, but not significantly, for patients who at month 24 had lost fewer than 15 letters from baseline vs. those who lost at least 15 letters (at 12 months: sham=1.8 and 2.9; 0.3 mg=-0.006 and 0.46; 0.5 mg=0.19 and 0.35 disc areas, for those who lost fewer than 15 letters and those who lost at least 15, respectively).2882
Researchers from
As a recap of the trials: In
With the 0.5-mg dose at two years, in the MARINA trial, 33 percent of patients gained more than 15 letters; in ANCHOR, 41 percent gained more than 15 and in PIER only 8 percent did this well. At least 6 percent of the patients gained more than 30 letters in the first year of the trials.
The researchers say the ranibizumab therapy was superior to PDT or sham in terms of letters gained, but the proportion of acuity gained was lower in the trial that used quarterly dosing.2883
Macular Edema Research
Though there are no results yet, researchers from the RIDE and RISE Phase-III, multicenter, controlled trials of intravitreal ranibizumab in macular edema with center involvement secondary to diabetes mellitus will discuss the trial design, rationale and its initial enrollment of patients. Each trial arm will enroll around 366 patients, and subjects will be randomized to intravitreal ranibizumab or sham injection at about 100 sites.1562
The PACORES group that's studying intravitreal bevacizumab for AMD is also studying the drug in patients with diffuse macular edema, and will share its 12-month follow-up at this year's ARVO meeting.
In the study, physicians reviewed the charts of 82 patients (101 eyes) with diffuse DME who received either 1.25- or 2.5-mg intravitreal injections of bevacizumab. The study abstract doesn't specify how many patients were in each group. The average number of injections per eye was three (r: one to 10) over a 12-month period.
At one year, in the 1.25-mg group, best-corrected acuity increased by 2.9 ±3.6 lines (p<0.005); it increased by 0.7 ±5.5 lines in the 2.5-mg group (p<0.05). In the 1.25-mg group, the mean central macular thickness decreased from 419 ±201 µm at baseline to 268 ±95.5 µm at a year (p<0.0001). It decreased from 388 ±162 µm to 296 ±114 µm in the 2.5-mg group (p<0.0001). Adverse events included transient hypertension in one patient (1.2 percent), transient increased IOP in one eye (1 percent) and tractional retinal detachment in one eye (1 percent).1563
Mark Gillies, MD, PhD, of the
Of 43 patients in the study, 34 eyes were randomized to receive treatment while 35 received placebo. Five-year data is available on 52 of the eyes (75 percent). After two years, all patients, including those receiving placebo, received triamcinolone injections if there was persistent visual impairment with central macular thickness greater than 275 µm.
Dr. Gillies reports that, during the study's third year when all eyes were eligible for treatment, 41 percent of the treatment eyes (12 out of 29 eyes) and 36 percent of the placebo eyes (10 out of 28 eyes) warranted triamcinolone injection. In the treatment group, the mean visual acuity score increased from 59.2 at baseline to 62.8 letters at five years. In the placebo group, it decreased from 59.7 to 58.3 letters. The mean reduction in central macular thickness was 76 µm in the initial treatment group vs. 186 µm in the initial placebo group. Eighty-eight percent of the initial treatment group (23/26 eyes) required glaucoma meds at some point vs. only 19 percent (5/26 eyes) of the initial placebo group. Three of the treatment eyes needed trabeculectomies, and there was one case of infectious endophthalmitis in the treatment group. Also, 73 percent (19/26 eyes) of the initial treatment group underwent cataract surgery, compared to only 8 percent (2/26 eyes) of the initial-placebo group.1565
Researchers from
In this prospective, double-masked, controlled trial, 90 subjects with previously untreated DME were randomly assigned to receive laser photocoagulation by the modified ETDRS (30 eyes), normal (29 eyes) or high-density (31 eyes) micro-pulsed technique (delivered with the Opto FastPulse Laser; Opto,
Central macular thickness decreased by an average of 97 µm in the modified ETDRS group, by 33 µm in the normal density sub-threshold micro-pulsed technique and by
146 µm in the high-density sub-threshold micro-pulsed technique. At 12 months, the mean change in visual acuity was zero letters in the modified ETDRS group, three letters worse in the normal-density group and two letters better in the high-density group.
The researchers say that their results don't support the use of sub-threshold laser treatment of DME, but imply a short-term clinical performance benefit to a high-density technique when compared to a modified ETDRS technique. They add that the precise role of sub-threshold laser treatment may be better defined as users formulate optimized treatment guidelines and perform more comprehensive trials.1566
Researchers from various sites around the
Rapamycin (Sirolimus) is a specific inhibitor of the mammalian target of rapamycin (mTOR), a regulatory protein kinase. The researchers say it has a broad activity that includes anti-inflammatory, anti-permeability and anti-proliferative effects. They say that inhibition of mTOR by rapamycin downregulates hypoxia-inducible factor 1 (HIF-1), a regulator of oxygen homeostasis that's elevated in the diabetic eye. The purpose of the study is to evaluate the effects of a proprietary liquid depot-forming formulation of rapamycin in DME patients.
In the study, 50 patients were randomly assigned to receive either a single intravitreal rapamycin injection of 44, 110, 176, 264 or 352 µg or a single subconjunctival injection of 220, 440, 880, 1,320 or 1,760 µg. The patients were followed for up to 180 days.
The researchers didn't observe any dose-limiting toxicities or serious ocular adverse events related to rapamycin. There was a statistically significant treatment effect (p<0.05) at 14, 45 and 90 days. Mean best-corrected acuity improvements were 8.8 ±4.4, 11.4 ±7.6, and 7.4 ±8.6 letters at 14, 45 and 90 days, respectively, following a single 440-µg subconjunctival injection. There were mean OCT reductions in central subfield retinal thickness of 33 ±60, 78 ±50, and 54 ±93 µm at 14, 45, and 90 days, respectively.
The researchers report similar changes following a single 352-µg intravitreal injection, with mean acuity improvements of 11.6 ±7.7, 6.4 ±7.1, and 7.8 ±2.2 letters, and mean OCT reductions of 72 ±53, 42 ±39, and 61 ±47 µm at 14, 45, and 90 days, respectively. These improvements persisted through 180 days.1567
Researchers from 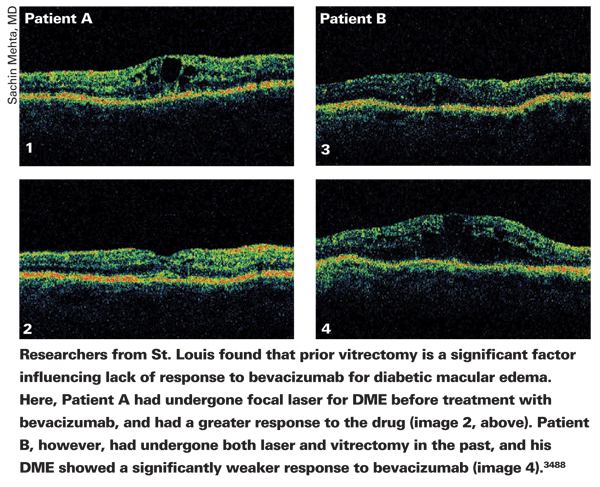
In the study, researchers reviewed 60 eyes of 54 patients who received 1.25 mg/0.05 ml intravitreal bevacizumab for refractory DME. The average follow-up was six months (r: three to 17 months). All of the eyes had undergone previous treatments, such as focal laser therapy (83 percent), sub-Tenon's triamcinolone (22 percent), intravitreal triamcinolone (47 percent), full-scatter panretinal laser therapy (40 percent) and vitrectomy (28 percent).
At the final follow-up visit, mean visual acuity had improved to 0.65 ±0.3 logMAR (a little worse than 20/80) from 0.71 ±0.28 logMAR (a little worse than 20/100) (p=0.034). Of the eyes that had an improvement in acuity of at least one Snellen line, significantly fewer had undergone prior vitrectomy (p=0.0169) when compared to eyes with no improvement or worsening of acuity.
The baseline mean CRT was 440 ±106 µm, and mean CRT after treatment was 386 ±129 µm (p=0.0077). The only baseline characteristic predictive of a CRT reduction of 50 µm or more was phakic lens status. There wasn't a significant relationship between improvement in acuity and CRT decrease.3488
Odds and Ends
A masked, randomized trial from
Researchers randomized 16 eyes with chronic CSC into two groups to receive either PDT with verteporfin (fluence: 25 J/cm^2, light dose rate: 300 mW/cm^2) or no therapy. The inclusion criteria were as follows: best-corrected visual acuity between 0.2 and 1 logMAR (20/32 and 20/200); presence of subretinal fluid and/or serous pigment epithelial detachment on OCT without regression for three or more months; RPE leakage on fluorescein angiography and choroidal vascular hyperpermeability on confocal scanning laser indocyanine green angiography. Exclusion criteria were: any previous treatment for CSC; evidence of other chorioretinal disorders; media opacities; and treatment with systemic steroids. The physicians applied the laser irradiation on the areas of choroidal vascular hyperpermeability as observed on ICGA. They used non-confluent laser spots in cases of multiple hyperpermeable areas, including the fovea if it were involved.
There were no significant changes in any outcome in the control group. However, in the treatment group, there was a significant improvement in far and near best-corrected vision in comparison with both baseline (p=0.008 for far and 0.000 for near), and the control group (p=0.010 far and p=0.000 near), with the greatest effect at week 24. In all eyes treated with low-fluence PDT, there was a complete resolution of subretinal fluid with a significant reduction of central macular thickness. At week 24, in treated eyes, there was a significant improvement in mean fixation stability. No recurrence was seen during the follow-up period. No adverse events occurred in any treated patients.3278
Researchers from centers in
In the study, researchers injected a viral vector expressing human RPE65 cDNA under the control of a human RPE65 promoter in four patients with early onset severe retinal dystrophy caused by mutations to the RPE65 gene. So far, there have been no complications associated with the surgical delivery of the vector. The researchers say they haven't detected any systemic dissemination of the viral vector genome and no evidence of immune responses to rAAV vector capsid or RPE65 proteins. There's been no adverse effect on retinal function.1133
Researchers from the Cleveland Clinic say that varying the oxygen levels based on a baby's age decreases the severity and prevalence of retinopathy of prematurity.
A database prospectively recorded the gestational age, birth weight, ROP stage and ROP zone of infants. The researchers then retrospectively analyzed the data over two years.
The first year of these two-year exams was performed on children with standard oxygen supplementation (saturation target >95 percent). The researchers performed the second-year exams on infants whose target saturation was 85 to 92 percent when younger than 34 weeks and 93 to 97 percent when older than that.
Physicians examined a total of 190 consecutive patients between September 2005 and October 2007, 98 of whom were examined prior to and 92 who were examined after the change in oxygen standards. Mean gestational age was 28 weeks and mean birth weight 1,085 g for both years. Two hundred and 155 exams were performed on the standard oxygen and the changed-oxygen groups, respectively. ROP was present in 35 percent of infants who underwent standard oxygen supplementation, compared to 13 percent in the year after the change in oxygen standards. Also, threshold disease decreased from 7 percent to 1 percent between the years. There was a decrease in all pathogenic stages of ROP from pre- oxygen change to post: Stage 1 ROP: 20 percent vs. 12 percent; Stage 2: 12 percent vs. 3 percent; Stage 3: 10 percent vs. 2 percent. The researchers also found an increase in Stage 0 (immature vessels) and Zone 3 (fully vascularized retina) after the oxygen change. Before the change, 24 percent were Stage 0 vs. 43 percent after the change. Twenty-three percent had fully vascularized retina before the change, while 30 percent were fully vascularized after. The increase in Stage 0 or normal retinal development was matched by a decrease in less mature zones, implying that the retina tended to develop at a quicker rate within the new oxygen parameters. There were no Stage 4 or 5 eyes.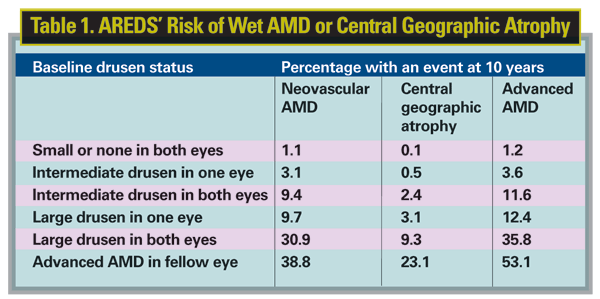
The researchers say that meticulous control of oxygen, with comparatively lower oxygen targets at early gestational ages and higher oxygen limits at older ages, decreased the severity and prevalence of ROP.5045
Researchers from the Age-related Eye Disease Study have done an analysis of the 10-year estimated rate of progression to neovascular AMD based on baseline drusen size and adjusted for covariates.
In AREDS, 4,642 patients were followed for a minimum of 10.5 years. Based on how those patients progressed, researchers developed the probabilities of a patient developing wet AMD or central geographic atrophy that appear in Table 1.5065
Dr. Regillo is professor of ophthalmology at











