Thanks to the development of spectral-domain optical coherence tomography, the scope of information attainable by OCT has expanded dramatically. Use of this technology as an aid to glaucoma management is still in its early stages, but the advantages of using it for this purpose are becoming clearer every year.
Here, I'd like to profile some of the ways our group has found SD-OCT to be helpful when monitoring glaucoma patients. Most of these approaches are possible using some or all of the SD-OCT instruments currently available; one particularly useful technique requires additional software that should be available soon.
Scanning the Anterior Segment
Anterior segment imaging can be performed by various currently available SD-OCT machines. Glaucoma applications include:
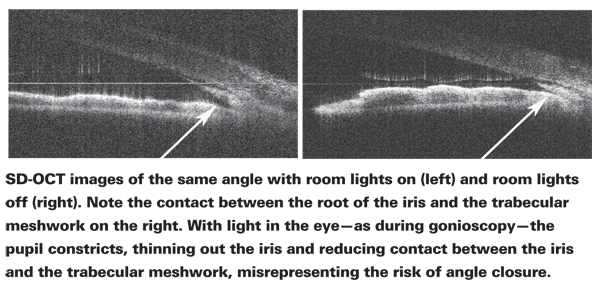
• Being able to study the angle without incident light. I believe this is the most advantageous use of this technology. When we use gonioscopy to check the condition of the angle the slit-lamp light constricts the pupil, pulling the iris away from the angle.
This can make the angle appear open—even if it's not open when ambient light is minimal. In contrast, SD-OCT uses the infrared spectrum, so the pupil doesn't constrict during the scan. Furthermore, many doctors are not comfortable doing gonioscopy; SD-OCT provides a way to evaluate the angle that's objective and far easier to do.
When you see the angle in its natural state, you'll be surprised how many patients who might be diagnosed as having open-angle glaucoma actually have narrow angles that are occludable; lens-induced (phacomorphic) narrow angles are as common as the pupillary block mechanism. We've discovered that many apparent cases of OAG in our practice really aren't; the diagnoses were simply a result of the technology limitations we've had to work with.
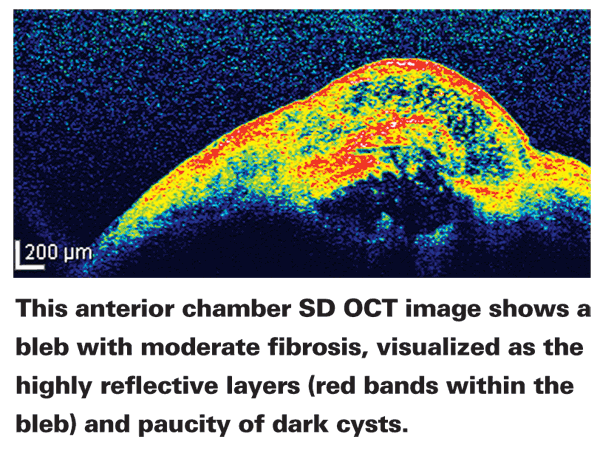
• Monitoring the filtering bleb. Anterior segment OCT allows us to take a cross-section of the bleb to see its status in terms of scarring and fibrosis. This makes it possible to change the medical treatment of these blebs, based on how they're healing.
• Studying the trabecular meshwork and Schlemm's canal. Being able to see the details of these anatomical features can help with both our understanding of the pathophysiology of glaucoma and with monitoring the status of some of the newer glaucoma surgeries. Canaloplasty, the Trabectome, the Aqueous Shunt, Glaukos' trabecular bypass shunt, excimer laser trabeculostomy—all of these can be easily studied by cross-sectioning the detailed area.
For example, we're very happy when the Trabectome works, but when it doesn't work we don't know why. Did the trabecular meshwork scar down? Did the iris block it? With a cross-sectional instrument, we can see why the surgery succeeded or failed. Perhaps after using the Trabectome in a given patient, pilocarpine is needed to keep the iris away.
With anterior segment SD-OCT we can see that. This technology may even help surgeons to design new glaucoma surgeries.
• Seeing the status of glaucoma-related factors. This technology makes it easy to monitor the condition of plateau iris syndrome, synechiae and other issues.
Despite the fact that anterior segment imaging with SD-OCT can be done using a number of currently available instruments, few clinicians are doing so—in part because they're not aware of the technology's potential, and in part because this use is not yet reimbursable. However, some crucial information—such as the true condition of the angle—can only be determined with this technology. Fur-thermore, it's a great demonstration tool. Many of these conditions are highly asymptomatic, making it difficult to demonstrate to the patient that the angle is narrow and needs a treatment such as laser iridotomy. With SD-OCT, you can show the angle on-screen, first with the room lights off, then with the lights on.
Glaucoma and the Macula
Macular thickness is a potentially useful measurement for detecting glaucoma and following progression because that's the area in which most of the ganglion cells are being lost. More than half of all ganglion cells are in the macula, and the nerve fiber layer and ganglion cells together comprise about 40 percent of the retinal thickness. In addition, barring other diseases of the macula such as macular degeneration or diabetic retinal edema, macular thickness doesn't change over time. So if thickness is lost, the diagnosis is typically glaucoma. And these losses are sometimes seen many years before visual field defects become apparent.
The concept of using macular thickness for monitoring glaucoma was introduced in the late 1990s using an instrument called the retinal thickness analyzer. The RTA was able to provide us with a macular thickness map; for the first time, that allowed our group to describe retinal thickness in normals.1 We then showed that macular thickness was very much affected in glaucoma—in many cases much earlier than could be detected with visual fields.2
One reason that it makes sense to monitor macular thickness is that the ganglion cell population in the macula—unlike the total number of ganglion cells in the entire retina—is relatively stable among normals.3 In contrast, the optic nerve varies quite a bit in terms of size, shape, sloping, etc.; the nerve fiber layer is somewhere between the optic nerve and macula in terms of being similar among normals. So the macula is ideal for comparison to a normative database. Furthermore, because the ganglion cells represent a huge portion of the thickness of the retina, changes in macular thickness are much easier to detect than changes in a very thin layer such as the NFL.
Unfortunately, macular thickness has been difficult to measure accurately. Zeiss's time-domain OCT, Stratus, did not capture macular thickness in sufficient detail to be of help to glaucoma specialists. The measurements were far inferior to measuring the nerve fiber layer, so macular thickness as a means to follow glaucoma progression never became popular.
Measuring the Macula with SD-OCT
The advent of SD-OCT technology revived the possibility of using macular thickness as a glaucoma marker because SD-OCT technology gives us much more detailed information with each cross-section than time-domain OCT could. In addition, because of SD-OCT's high speed, accurate measurements of large areas of the retina can be obtained. Nevertheless, its ability to make fine measurements has been hindered by microsaccades, which can cause inaccurate measurements and low reproducibility.4,5 However, the Spectralis SD-OCT from Heidelberg includes eye-tracking capability, which may reduce the error resulting from microsaccades during image acquisition.6 The Spectralis also allows for customization of volume scanning protocols via adjustment of scan density and the dimensions of the scan area.
To take advantage of customizable scan protocols, our group has been working with
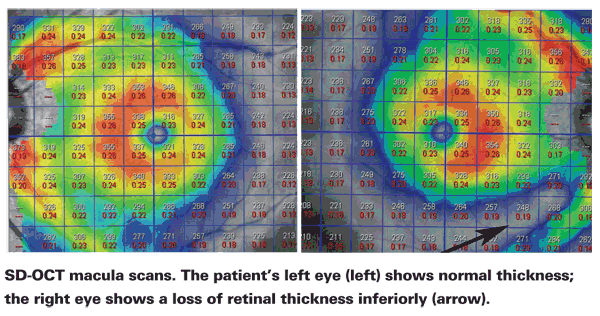
This development makes it feasible to use this technology to follow macular thickness changes in glaucoma patients. In fact, we've found that these macular thickness measurements are allowing us to pick up nerve fiber layer and retinal thickness losses before the visual field shows any abnormality. These measurements should also be useful when patients have lost a great deal of tissue, making it difficult or impossible to monitor them using visual fields. There will still be tissue remaining in the macula that will allow us to monitor whether the patient is getting worse or our treatment has been successful.
As with any technology, artifacts can occur; problems such as epiretinal membranes, diabetic macular edema and macular degeneration can throw off macular thickness measurements. But in those situations, we can use retinal nerve fiber layer thickness measurements.
Unfortunately, it will take time to accumulate data proving that this strategy really is an effective way to monitor glaucoma patients. So far, we have proof that macular thickness changes as the disease progresses, and that we can pick up very early tissue losses before visual field changes take place. The fact that reproducibility is high gives us hope, because high reproducibility means that if we take two measurements six months or a year apart and find change, we can be confident that it's real change.
This kind of macular thickness measurement may help us follow patients objectively, rather than having to depend entirely on visual fields. I'm not saying that visual fields will be superseded any time soon; but this could provide another measure that's objective, and would help us interpret visual field changes more effectively. In the meantime, our group is assembling a normative database of macular thickness for both adults and children. We expect that project to be completed in six to eight months.
Should You Buy One?
If you're managing glaucoma pa-tients and you don't already own an SD-OCT instrument, I think it's worth considering as a purchase. It's a significant revolution in technology, and it's clearly advantageous to be able to monitor both the anterior and posterior segments. However, I wouldn't abandon other instruments such as the GDx and HRT3, which have been proven to be useful in glaucoma. Furthermore, I'm not sure having multiple technologies is necessary at this time.
The new macular thickness software for the Spectralis isn't available to the public yet, but we hope it will be before the end of 2010. This software will be proprietary to the Spectralis, but other SD-OCT instruments have the potential to accomplish this as well; there's nothing unique about image registration or changing the color scale.
Of course, it's early in the game. We don't yet know whether OCT will live up to its promise as a follow-up tool. But if you're thinking about switching from a GDx or HRT, this would be a good technology to consider.
Dr. Asrani is associate professor of ophthalmology in the Glaucoma Service at
1. Asrani S, Zou S, D'Anna S, Vitale S, Zeimer R. Noninvasive mapping of the normal retinal thickness at the posterior pole. Ophthalmology 1999;106:269-273.
2. Zeimer R, Asrani S, Zou S, Quigley H, Jampel H. Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping. Ophthalmology 1998;105:2:224-31.
3. Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol 1990;300:1:5-25.
4. Schuman, J. Spectral domain optical coherence tomography for glaucoma (an AOS thesis).Transactions of the American Ophthalmological Society 2008;106:426-58.
5. Han IC, Jaffe GJ. Evaluation of artifacts associated with macular spectral domain optical coherence tomography. Ophthalmology 2010. In Press.
6. Han IC, Jaffe GJ. Evaluation of artifacts associated with macular spectral domain optical coherence tomography. Ophthalmology 2010;in press.











