
A very energetic and outgoing 84-year-old white female presented in February 2005 complaining of blurry vision O.U. that had progressed gradually during the past six months. She reported that her last eye examination had been approximately two years earlier, at which time she was told she had cataracts. Her family history was remarkable for a sister with non-angiogenic (dry) macular degeneration.
Her current medications included Macrobid (nitrofurantoin, Procter & Gamble Pharmaceuticals) for a concurrent urinary tract infection, Toprol (metoprolol, AstraZeneca) for hypertension, 500mg acetaminophen p.r.n., and vitamins daily. She reported an allergy to penicillin-based antibiotics.
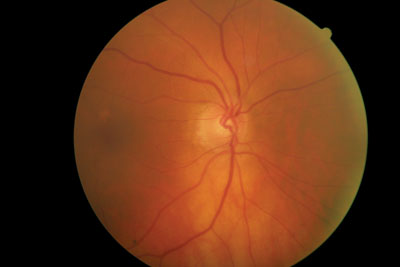
This 84-year-old patient has neuroretinal rim loss inferiorly in the right eye. Should we treat her or monitor her?
Diagnostic Data
Entering visual acuity was 20/30 O.U. Best-corrected acuity was 20/25+ O.U. through hyperopic astigmatic correction. There was approximately a -0.75D shift in each eye to achieve this best-corrected acuity.
Pupils were equal, round and reactive to light and accommodation, with no afferent pupillary defect. Extraocular motilities were full in all positions of gaze.
A slit lamp examination of her anterior segments was remarkable for a moderate amount of bilateral dermatochalasis that did not interfere with vision. Also, corneal arcus was present bilaterally, along with scattered endothelial guttata O.U. Her anterior chambers were deep and quiet, and her angles were graded as 2+ by the Von Herrick method. Applanation tensions were 22mm Hg O.D. and 21mm Hg O.S. at 11 a.m.
Through dilated pupils, her crystalline lenses were characterized by grade 2 nuclear and grade 1 cortical cataracts, which were relatively symmetric between the eyes.
Her cup-to-disc ratios were 0.50 x 0.65 O.D. and 0.45 x 0.45 O.S. The neuroretinal rim in the right eye was thinned from 6 to 8 oclock. Her discs were of normal size. Her macular evaluation was unremarkable, with only a loss of the foveal reflex in both eyes and retinal pigment epithelium window defects. Her peripheral retinal examination was also unremarkable, and there were bilateral posterior vitreous separations.
The patient returned for a full glaucoma workup four weeks later.
At this time, intraocular pressure measured 22mm Hg in each eye. Central corneal thickness (CCT) measured 504m O.D. and 502m O.S. Gonioscopy demonstrated grade 3 open angles O.U. with moderate amounts of trabecular pigmentation. Threshold visual fields had poor reliability characteristics and were essentially uninterpretable. Optic nerve imaging confirmed neuroretinal rim loss inferiorly O.D. and called into question rim characteristics in the fellow eye at 5 oclock.
Diagnosis
Given her asymmetric cups, the thinned neuroretinal rim O.D., and the relatively thin CCT in both eyes, I diagnosed early glaucomatous optic neuropathy that was greater O.D. than O.S., and initiated a trial of Lumigan (bimatoprost, Allergan) 1 drop O.U. h.s.
Subsequent visits to the present day have demonstrated IOPs averaging 13mm Hg O.D. and 12mm Hg O.S. while medicated with Lumigan. Reliable threshold fields were obtained on three occasions, and they show early glaucomatous field loss, again greater O.D. than O.S. The field loss has not progressed in either eye. Subsequent optic nerve images have shown no further progression of her neuroretinal rim loss O.U.
Discussion
On the surface, this case seems relatively straightforward: A patient presents with elevated IOP, optic nerve damage, visual field loss and thin CCT, and treatment with Lumigan has stabilized the visual field and nerve tissue loss.
Looking more closely, though, one could argue whether the costs of medicating this patient outweigh her likelihood of developing impaired vision, especially given her age. Essentially, were asking: Will the patient die before her vision becomes impaired, or will she suffer vision loss before she dies? While that may sound callous, that is exactly what we need to think about before initiating treatment. In essence, how does our therapeutic plan affect the patients quality of life?
Several factors played a role in my decision to prescribe medication rather than passively monitor the patient. My primary consideration: her healthy, active lifestyle. At 84, she was quite active and animated (and now, at 86, she still is). The potential effects of vision loss on her lifestyle could be devastating.
Notice my choice of words: I said, Could be. The truth is, we will never know for certain what would happen if we chose the other treatment option: passive monitoring. Still, we must consider the facts before usboth those things specific to the eye (CCT, IOP, etc.) and ancillary pieces of the glaucoma puzzle (lifestyle, overall health, mobility, life expectancy, etc.)and formulate a game plan based on those facts.
Another factor involved in the decision to treat this patient is her medicated blood pressure. Average BP readings in the office are 110/68. Blood pressure variations, as well as episodes of nocturnal systemic hypotension, can play a part in the development and progression of glaucomatous optic neuropathy, especially in situations of pressure-independent glaucoma.1 While not all patients have blood pressure variations, elderly patients tend to have more episodes of blood pressure instability. I believe that using a beta-blocker to treat her hypertension would have put her at additional risk of progressive, and perhaps rapid, field loss, especially since her blood pressure was somewhat low.
Although I chose to initiate therapy, one can argue that monitoring her instead would be appropriate. Would it have been significantly less costly to the patient had I chosen this option? I dont think so. The only substantial cost difference between monitoring this patient vs. intervening therapeutically would be the costs associated with the medication itself. Had I chosen to monitor her, I would still see the patient at the same intervals, and I would still monitor visual fields, optic nerves and IOP at the same frequency as if she was medicated. The only difference would be the cost of medication. Furthermore, there are various patient assistance programs to help patients who cannot afford their medications obtain them at little or no cost. (See Glaucoma Patient Assistance Programs, below.)
Glaucoma Patient Assistance Programs RxHope: http://rxhope.com/ Alcon Cares: 1-800-222-8103 Allergan: 1-800-553-6783 Merck: 1-800-727-5400 Pfizer Connection to Care: 1-866-776-3700 Vistakon Pharmaceutical: 1-866-815-6874
Could it have been more costly NOT to intervene in her care? Perhaps, but we will never know. Interestingly, a study in the Netherlands showed that simply taking IOP readings during routine visits is the most cost-effective method in reducing glaucoma-related blindnesssomething optometrists in the United States already do regularly.2 (Its also interesting to note that in some European countries, tonometry is not routinely performed or performed only in high-risk patients.) In a similar vein, investigators recently reviewed current peer-reviewed published studies and found that even today, some studies fail to evaluate CCT in patients who have ocular hypertension.3
Fortunately, most optometrists in the
1. Gramer EJ. Role of Nocturnal Hypotension in Glaucomatous Visual Field Loss (poster #522). Presented at the
2. Peeters A, Schouten JS, Webers CA, et al. Cost-effectiveness of early detection and treatment of ocular hypertension and primary open-angle glaucoma by the ophthalmologist. Eye 2006 Nov 24; [Epub ahead of print].
3. Tavares IM, Medeiros FA, Weinreb RN. Inconsistency of the published definition of ocular hypertension. J Glaucoma 2006 Dec;15(6):529-33.











