
Few things provoke more anxiety (for both the patient and the doctor) than noticeable visual field loss. The underlying diagnosis is often serious and can threaten long-term vision and even the patients well-being. Glaucoma is the leading cause of visual field loss across all age groups.1 However, glaucoma patients often dont present with an initial chief complaint of visual field loss, unless the disease is quite advanced.
This review discusses conditions (other than glaucoma) that can cause symptomatic visual field loss, and it includes a practical guide to ancillary testing and referral of these patients.
The Visual Pathway
To fully appreciate the potential underlying causes for various visual field defects, it is important to have a working knowledge of the anatomy of the visual system (figure 1).
Remember: Nearly half of the nerve fibers cross at the optic chiasm, so the loss of visual field on one side is quite different than loss of vision in one eye. Patients are often unable to accurately determine the location of field loss. So, if the patient says, I cant see on the left side, you need more clarification before you develop a short list of differential diagnoses.
Also, defects that respect the vertical midline are nearly always located at the chiasm or farther back in the visual pathway. Causes of a visual field defect that respects the horizontal midline (altitudinal visual field defects) are more frequently located anteriorly in the pathway (near the optic nerve or retina).
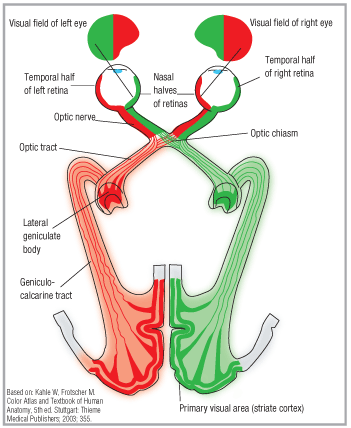
1. The visual pathway. Note that half of the optic nerves cross at the optic chiasm, causing vision loss on one side to be quite different than that in one eye.
Demographics of VF Defects
Nearly one out of every 20 people past age 55 has visual field loss that is significant enough to impair daily functioning.1 Difficulties experienced by these patients include a higher risk of falling, reduction in physical mobility, disability, diminished quality of life, and diminished ability to watch television or read.1-7 The incidence of visual field loss increases with age. One study found a five-fold increase of visual field loss between ages 55 and 80.8
An even more surprising finding: In one study of 61 post-stroke patients suffering from homonymous visual field loss (as determined by Humphrey visual field analysis), none were aware of the visual field loss.9 Also, only two of those 61 patients were identified as having field loss using the National Institutes of Health Stroke Scale (NIHSS) technique, which is a finger-counting confrontation visual field screening. To further complicate matters, 30% of those subjects with significant visual field loss were still driving.9
Red Eye, Red Herring A 49-year-old Hispanic male presented with complaints of a red, irritated left eye with significantly reduced vision in the same eye. He was alert and oriented, had a normal gait and displayed no affective abnormalities. Vision was 20/30 O.D. and 20/200 O.S. O.S. left, O.D. right. Note the bilateral involvement despite unilateral (O.S.) complaints.
Causes of Visual Field Loss
Color vision, visual fields and extraocular motilities were normal in both eyes. A trace relative afferent pupillary defect was seen O.S., but both pupils were of normal size. Visual fields are below. Ultimately, this patient was diagnosed with neurosyphilis, and was treated with penicillin. His visual acuity recovered fully, but his visual fields only partially recovered. 
Glaucoma is the leading cause of field loss across all adult age groups when tested using automated threshold or supra-threshold perimetry.8
Other leading causes of visual field loss in patients past age 55 are non-glaucomatous optic nerve disease, stroke, age-related macular degeneration (AMD) and retinal vascular occlusive disease.1,8 Stroke is the leading cause of homonymous hemianopias, followed by trauma, tumor, brain surgery and demyelination.10
In younger age groups, prevalence of visual field loss is much lower, and causes are more varied and may be functional or psychogenic in nature. Tumors; drug-related; hereditary disease of the macula, retina or optic nerve; demyelination; and intracranial hypertension are among the more common causes.11-15
Patient Evaluation
When a patient presents complaining of loss of some or all of the vision in one eye or in a particular part of the field of vision, the most critical step is to determine if this is a neurological emergency. Is the patient alert and oriented? Are there any other sudden-onset neurological changes?
If the patient displays disturbances in speech, gait, memory, facial symmetry or behavior, an immediate referral to the ER is essential. Such changes, among others, may indicate a cerebrovascular accident (stroke). Time is critical! Dont waste precious minutes before sending the patient for evaluation and care.
If the patient is alone, calling an EMT ensures safe transport to the ER, but if the patient is accompanied by a family member or caregiver, instruct this person to take the patient to the ER with written instructions from you. Alert the triage personnel at the hospital that a potentially time-critical patient is on the way.
Aside from these rare but critical cases, the most important testone that must not be skippedis automated perimetry.
Before formal visual field testing, however, you must determine several things. Besides your normal history and examination sequence, ask:
Is the field loss in one eye, or is it on one side of the field? Confrontation visual field testing is not 100% reliable, even when done properly and carefully. You can enhance the reliability of standard finger-counting testing by adding a facial Amsler test (have the patient fixate on your eyes and ask him or her if your other facial features appear distorted or are missing) and by performing double simultaneous stimulation confrontation fields.
Did the loss occur suddenly or gradually? When did you first notice it? Vision flickering off and on is a common symptom of patients with giant cell arteritis. It occurs in the days or hours leading up to profound vision loss.
Often, patients first notice visual symptoms accidentally, even if the problem has been long-standing. Ask what the patient was doing when he or she first noticed the loss. A patient who experiences a large hole in the vision of the right eye immediately upon awakening may be quite different than the patient who noticed vision loss in one eye when the fellow eye was covered after a minor injury.
Has this ever happened before? Recurrent and transient holes in the visual field may indicate a vascular perfusion deficit. Very low blood pressure and/or low blood sugar can cause this type of symptom. Its not uncommon during pregnancy and puberty for patients to experience transient scotomas of this sort. Consultation with the appropriate medical caregiver is prudent.
When to do Automated Field Testing: Any complaint of headaches not explained by the exam. Any complaint of a fixed missing spot in the visual field (monocular or binocular). Identification of field loss using screening techniques. History of cerebrovascular accident. History of head trauma. Unexplained visual acuity loss. Optic nerve abnormalities. Any other clinical finding (subjective or objective) that does not correlate with other examination findings.
In many cases, attention to proper hydration and caloric intake, along with awareness of the effects of postural changes (e.g., standing up quickly from a seated or reclined position) is sufficient for managing the symptoms. In other cases, medical evaluation and treatment are necessary; vertebrobasilar insufficiency (VBI) and ocular ischemic syndrome (OIS) secondary to internal carotid artery insufficiency are far more serious potential causes of transient binocular (caused by VBI) and monocular (caused by OIS) scotomas.
Does the missing area or spot remain the same size, or does it change? Scotomas that change can be considered transient, so they are more likely vascular in origin. Fixed scotomas usually signal retinal, optic nerve, or neurological involvement.16
Are there any other unassociated symptoms? The presence or absence of coincident flashing lights, floaters, dyschromatopsia, eye pain, headache, vertigo, diplopia, metamorphopsia or other seemingly unrelated symptoms will often direct your management. A referral to a retinologist, neurologist or internist may be required.
Is there a relative afferent pupillary defect (RAPD)? Compare the afferent anterior pupillary pathway of one eye vs. the fellow eye. Slightly more than half the nasal pupil pathway fibers cross at the chiasm, so significant defects anterior to the chiasm cause an RAPD. Lesions in the optic tract can also cause an RAPD in certain instances.17
The bottom line: From a clinical standpoint, a right positive RAPD means that you should be looking for a problem in the right retina and/or optic nerve. If you find nothing, neuroimaging is essential; place emphasis on the retro-orbital space, the optic nerves, chiasm and optic tracts.
If you think you see an RAPD but you arent sure, verify your findings. After the patient has a chance to recover from the transilluminators bleaching effects, do a white light comparison test. Occlude the abnormal eye and shine the light in the normal eye. Ask the patient to assign a brightness value of 100 points to that light. Quickly switch the occluder to the normal eye, and shine the light in the abnormal eye. A value of 80% or less is considered clinically significant. Of course, this test should be interpreted relative to other potential findings, such as corneal edema, cataracts, or other conditions that can affect the patients perception of brightness.
Another way to help confirm subtle RAPDs is to purchase an inexpensive 0.3 log unit neutral density (ND) filter. This enhances subtle pupillary defects. Hold the ND filter over the normal eye, and shine the light through the filter. Depending upon the degree of the RAPD in the other eye, this may appear to even out the signals being carried in both afferent systems. This simulates a normal swinging flashlight test; in effect, the pupils appear to respond equally. Next, hold the ND filter over the suspect eye and repeat the test. If an APD is present, it will nearly always become much more obvious with the addition of the ND filter.18
Understand that the presence of anisocoria doesnt create a clinically observable pseudo-APD until anisocoria of 2mm or more is present.19 So, dont dismiss subtle or trace RAPD responses to a 0.5mm anisocoria.
Is there a color vision defect? In general, you can link the findings from color vision and pupil testing together. Optic nerve and significant retinal disease in one eye can create color vision disturbances and positive RAPD results in that eye. Whether you perform the dilated fundus examination before or after automated visual fields is your choice; you can find support for both sides of the issue.20-22 As long as the patients pupils are at least 3mm in diameter, you can probably evaluate the visual fields in these non-glaucoma cases regardless of the state of pupillary dilation. Just consider the cycloplegic effects of the dilating drops when determining which trial lens to use in patients who are not yet absolute presbyopes.
Are there other neurological signs? Depending upon your level of comfort, you may also want to perform a cranial nerve screening, or even a gait, balance and symmetry screening. If you are not comfortable performing such tests, you can refer this patient to a neurologist for them.
What does the rest of the ocular examination reveal? Carefully evaluate the anterior and posterior segments. Findings of ocular disease will dictate appropriate management strategies based upon the condition.
Common and/or Classic Visual Field Patterns and Their Causes Glaucoma, branch retinal artery occlusion, anterior ischemic optic neuropathy. Ring scotoma (usually bilateral). Temporal defect in one
Type of field defect
Sample visual field*
Common cause(s)
Altitudinal. These respect
the horizontal raphe, eithersuperior or inferior (unilateral or bilateral).

Total monocular field loss.

Prechiasmal lesion on the same side as the eye with the visual field defect.
Bitemporal field loss.

Lesion at or near the optic chiasm (not always a pituitary adenoma).

Retinitis pigmentosa.
Superior quadrantanopia.

Temporal lobe lesion (infarct, hemorrhagic stroke, trauma) on the side opposite the visual field defect.
Inferior quadrantanopia.

Parietal lobe lesion (infarct, hemorrhagic stroke, trauma) on the side opposite the visual field defect.
eye, central defect in the fellow eye.
*Images generated.Anterior chiasmal syndrome, or a lesion just anterior to the chiasm on the same side as the eye with the central field defect.
Patient Management
What if there is no ocular cause for the field loss? In such cases, refer the patient to a neurologistor better yet, a neuro-ophthalmologist.
The History Makes the Diagnosis A 46-year-old white male complained of blurry vision and a missing spot of vision in his left eye for approximately eight months. Vision was 20/20 O.D. and 20/80 O.S. At a visit in our clinic several months prior, vision O.S. had been recorded at 20/25. O.S. left, O.D. right. Note that the defect in the right (asymptomatic) eye strongly respects the vertical midline. This is considered a bitemporal visual field defect despite the extension into the superior nasal quadrant in the left field.
How quickly does the referral need to be made? When patients display other neurological symptoms, an immediate referral is required. Otherwise, many of these cases are considered to be urgencies rather than emergencies, and require referral within a few days to a week. How the actual referral is carried out will vary based on the patients insurance, availability of neurologists in your area and other factors.
There was a pronounced RAPD in the left eye with reduced color vision (1/14 plates) in the left eye only. White light comparison testing showed a 25% sensitivity in the left eye as compared to the right. A significant visual field defect was present in not only the left eye, consistent with the patients case history, but also in the right eye. The patient underwent neuroradiological evaluation, and a craniopharyngioma was found.
Unfortunately, during resection of the tumor, the right optic nerve was damaged, and the patient was left with partial visual field loss in both eyes.
The decision to order radiological testing or wait for the neurologist to do it will also vary, depending upon your working relationship with the neurologist and the imaging centers in your area. If you do order neuroradiology studies for your patient, its a good idea to work with the neurologist the first few times so that you dont end up with equivocal results. For example, I have had patients require a repeat scan because I didnt specify thin enough sections through a particular region. A normal scan sometimes turns out to be not so normal when re-evaluated with more critical parameters!
One exception to neurology referral is a patient with visual field loss that you suspect is psychogenic in origin. If you can confirm that the visual field is not organic in nature, careful consideration for a referral to a psychologist or psychiatrist is indicated.
Patients who present with an awareness of a loss of visual field need prompt and accurate diagnosis, as management often involves immediate further evaluation and intervention. Knowledge of the functional anatomy of neurological pathways is essential in the interpretation of examination results. Such knowledge will enhance proper triage of these often-critical patients.
Dr. Reed is an associate professor at
1. Ramrattan RS, Wolfs RC, Panda-Jonas S, et al. Prevalence and causes of visual field loss in the elderly and associations with impairment in daily functioning: the Rotterdam Study. Arch Ophthalmol 2001 Dec;119(12):1788-94.
2. Coleman AL, Cummings SR, Yu F, et al. Binocular visual-field loss increases the risk of future falls in older white women. J Amer Geriatr Soc 2007 Mar;55(3):357-64.
3. Freeman EE, Muoz B, Rubin G, et al. Visual field loss increases the risk of falls in older adults: the Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci 2007 Oct;48(10):4445-50.
4. Turano KA, Broman AT, Bandeen-roche K, et al. Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optom Vis Sci 2004 May;81(5):298-307.
5. Friedman DS, Freeman E, Muoz B, et al. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology 2007 Dec;114(12):2232-7.
6. McKean-Cowdin R, Wang Y, Wu J, et al. Impact of visual field loss in health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology 2008 Jun;115(6):941-48.e1.
7. McKean-Cowdin R, Varma R, Wu J, et al. Severity of visual field loss and health-related quality of life. Am J Ophthalmol 2007 Jun;143(6):1013-23.
8. Skenduli-Bala E, de Voogd S, Wolfs RC, et al. Causes of incident visual field loss in a general elderly population: the Rotterdam Study. Arch Ophthalmol 2005 Feb;123(2):233-8.
9. Townend BS, Sturm JW, Petsoglou C, et al. Perimetric homonymous visual field loss post-stroke. J Clin Neurosci 2007 Aug;14(8):754-6.
10. Zhang X, Kedar S, Lynn MJ, et al. Homonymous hemianopias: Clinical-anatomic correlations in 904 cases. Neurology 2006 March 28;66(6):906-10.
11. Lim SA, Siatkowski RM, Farris BK. Functional visual loss in adults and children: patient characteristics, management, and outcomes. Ophthalmology 2005 Oct;112(10):1821-8.
12. Barboni P, Savini G, Valentino ML, et al. Lebers hereditary optic neuropathy with childhood onset. Invest Ophthalmol Vis Sci 2006 Dec;47(12):5303-9.
13. Spencer EL, Harding GF. Examining visual field defects in the paedriatric population exposed to vigabatrin. Doc Ophthalmol 2003 Nov;107(3):281-7.
14. Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuron-oncology program. Neuro Oncol 2007 Oct;9(4):430-7.
15. Fishman GA, Bozbeyoglu S, Massof RW, et al. Natural course of visual field loss in patients with type 2 Usher syndrome. Retina 2007 Jun;27(5):601-8.
16. Barton JJS. The visual pathway from optic chiasm to striate cortex. In: Neuro-ophthalmology. Ed: Rosen ES, Eustace P, Thompson HS, et al.
17. Kardon R,
18. Kardon RH, Thompson HS. The pupil. In: Neuro-ophthalmology. Ed: Rosen ES, Eustace P, Thompson HS, et al.
19. Lam BL, Thompson HS. An anisocoria produces a small relative afferent pupillary defect in the eye with the smaller pupil. J Neuroophthalmol 1999 Sep;19(3):153-9.
20. Edgar DF, Crabb DP,
21. Kudrna GR,
22. Lindenmuth KA, Skuta GL, Rabbani R, et al. Effects of pupillary dilation on automated perimetry in normal patients. Ophthalmology 1990 Mar;97(3):367-70.











