 |
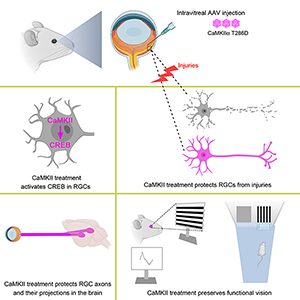
The researchers focused on retinal ganglion cells, which process visual information by sending images to the brain. These cells can degenerate as a result of retinal injury and retinal disease. The team of researchers demonstrated how reactivation of a key enzyme known as CaMKII and its downstream signaling in retinal ganglion cells through a gene therapy approach provided robust protection against further vision loss or impairment in multiple disease and injury models.
 | |
| Graphical Abstract |
“Neuroprotective strategies to save vulnerable retinal ganglion cells are desperately needed for vision preservation,” said senior author Bo Chen, PhD, associate professor of ophthalmology and neuroscience, and director of the ocular stem cell program at the Icahn School of Medicine at Mount Sinai. “We uncovered evidence for the first time that CaMKII is a key regulator of the survival of retinal ganglion cells in both normal and diseased retinas, and could be a desirable therapeutic target for vision preservation in conditions that damage the axons and somas of retinal ganglion cells.”
Glaucoma, the leading cause of irreversible visual impairment worldwide, affects 76 million people, some of whom will progress to blindness despite aggressive treatment to reduce the pressure in their eyes. The major barrier to restoring vision loss from glaucoma and other retinal diseases and injuries is that the long nerve fibers known as axons, which allow retinal ganglion cells to process visual information by converting light that enters the eye into a signal transmitted to the brain, do not regenerate.
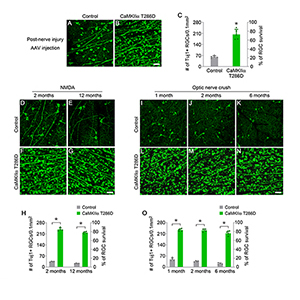 | |
| Reactivation of CaMKII provides post-injury and long-term RGC protection after excitotoxic or axonal injuries |
The team learned that CaMKII regulated the survival of retinal ganglion cells across many of these pathologies, and that in the small-animal excitotoxicity model, insults to the retinal ganglion cell’s somas or optic nerve injury to its axons led to inactivation of CaMKII and its downstream signaling target CREB (or cAMP response element binding protein). “Intriguingly, we found that reactivation of CaMKII and CREB provided robust protection for retinal ganglion cells, and that CaMKII-mediated protection slowed down the disease progression in both glaucoma models,” noted Dr. Chen, who is also the McGraw Family Vision Researcher at Icahn Mount Sinai.
That reactivation was made possible by a gene therapy approach deployed by the researchers to introduce a more active type of CaMKII into the original retinal ganglion cells to boost their activity. The modified version of CaMKII, with a mutated amino acid, was transferred to the targeted cells through an adeno-associated viral vector, a Food and Drug Administration-approved gene delivery system common to the growing field of gene therapy.
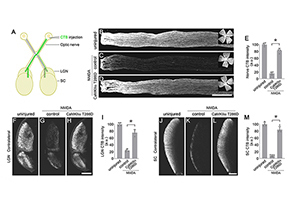 | |
| CaMKII reactivation protects RGC axons and their target projections to the brain |
Mount Sinai has filed patent applications for this technology through Mount Sinai Innovation Partners (MSIP), the commercialization arm of the health system. MSIP is in active discussions with multiple companies to help advance this treatment to the clinic.
The Mount Sinai study is supported by the National Eye Institute, which is part of the National Institutes of Health, along with the Research to Prevent Blindness and The Harold W. McGraw, Jr. Family Foundation.











