Neurologists and patients with multiple sclerosis are on the horns of a dilemma: How to best employ new disease-modifying drugs that are potent enough to induce life-changing remissions and too new to have amassed long-term safety data. Before 2010, when fingolimod was approved, such a quandary was unthought-of. Patients with relapsing-remitting MS had two options: beta interferons and glatiramer acetate or natalizumab. Now, with the addition of alemtuzumab, ocrelizumab and other high-potency monoclonal antibodies, there are more options offering more hope – and posing more questions. |
|
The advent of these powerful drugs poses yet another MS treatment dilemma: when should the approach be induction or escalation therapy? |
| The aim of this strategy is to prevent early structural damage related to inflammatory-mediated demyelination and axonal loss. |
Which patients are the most appropriate candidates?There are no formal guidelines designating which patients are most appropriate for induction therapy. But some papers have discussed common clinical characteristics that can help guide a decision.In the Journal of Neurology last year, Gabriel Pardo, MD of the Oklahoma Medical Research Foundation, and David E. Jones, MD of the University of Virginia described some characteristics of patients suitable for induction treatment: |
|
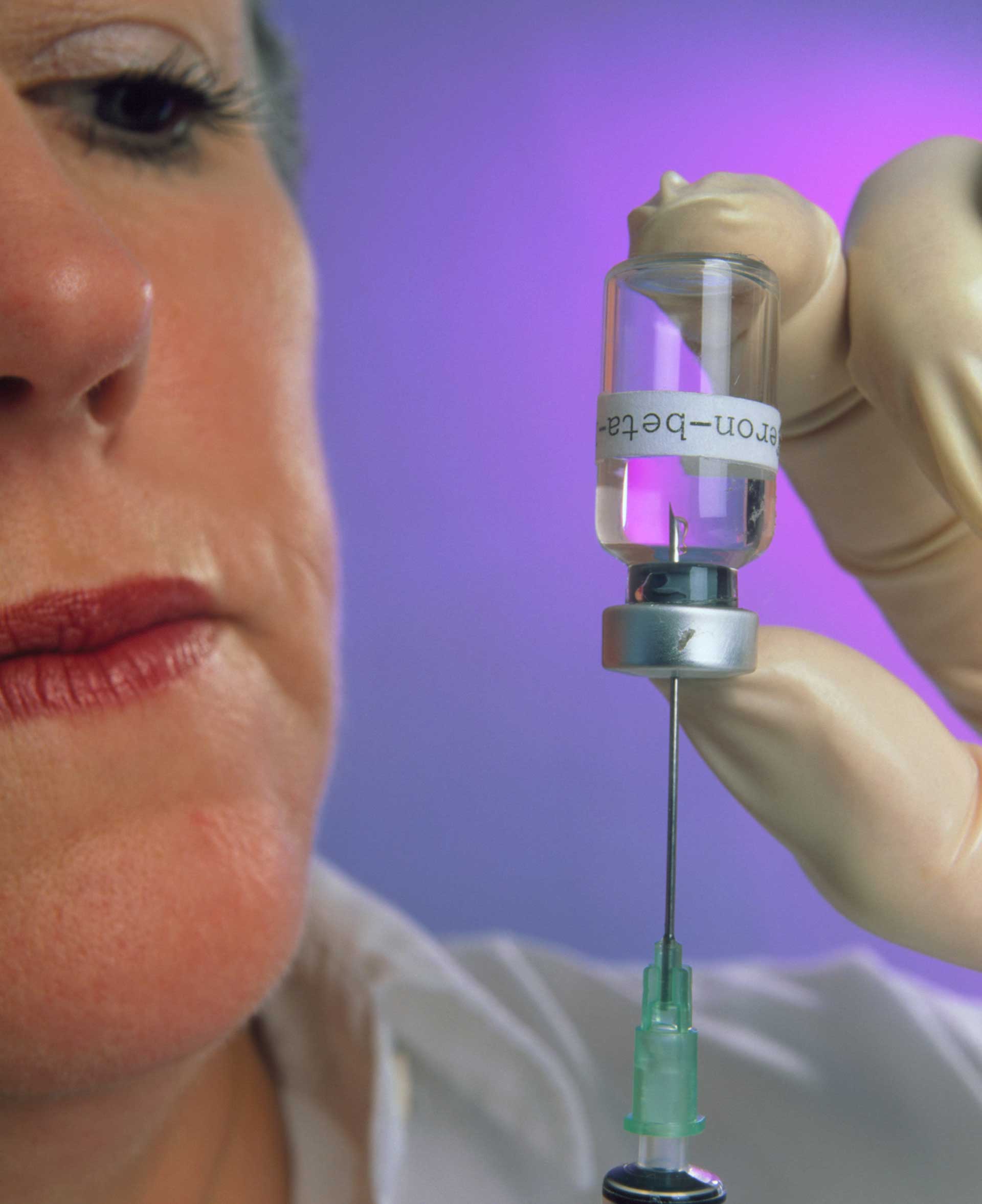 Credit: James King-Holmes/Science Source Daily injections of beta-interferon help to halve the number of attacks in people in the early stages of MS. This treatment, although expensive, offers hope and relief to MS sufferers while scientists try to find a cure for MS. |
Dr. LePage agreed, for the most part, writing that candidates should have a diagnosis of pure relapsing MS; young age; highly active disease with at least 2 relapses in the prior 12 months; severe relapse resulting in an EDSS score of 4 or more; an EDSS score increase of at least 2 points in the previous 12 months, and at least two enhancing lesions. Dr. Rinker doesn’t subscribe to any algorithm. “There’s no single factor that will tip me one way or the other. In most cases it ends up being an individualized decision. I try to make a two-way discussion with patients. I listen to their story and I gauge what kind of effects MS has had on them so far. Is this someone with only intermittent rare symptoms followed by good recovery, without a lot of lesions or residual disability? Then I would probably be more inclined to value safety over efficacy” when planning the therapeutic approach. “But someone else of the same age, who has had one or two attacks and didn’t recover fully or who has residual symptoms affecting ambulation or use of a limb, or who has a heavy disease burden on imaging, I’m going to be much more concerned that this is an active inflammatory version of MS. In those cases, I may be more inclined to start with a more aggressive therapy or start with a first-generation therapy but have a low threshold to escalate to something more aggressive.” Patient desires also play a big role in the decision, he said. “You have to consider the risk tolerance of each patient. Often, they have more fear of the adverse effects of a medicine than of the MS itself. I can’t really explain that, but sometimes I find myself making the argument that their illness may be scarier than the treatments. This can be a process, getting a patient from being skeptical about treatment to believing the treatment can help, and that it is worth the potential side effects.” |











